Engineering memory T cells as a platform for long-term enzyme replacement therapy in lysosomal storage disorders
- PMID: 39367605
- PMCID: PMC11573576
- DOI: 10.1016/j.ymthe.2024.09.033
Engineering memory T cells as a platform for long-term enzyme replacement therapy in lysosomal storage disorders
Abstract
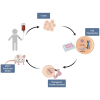
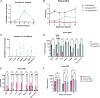
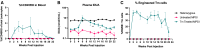
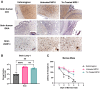
Enzymopathy disorders are the result of missing or defective enzymes. Among these enzymopathies, mucopolysaccharidosis type I is a rare genetic lysosomal storage disorder caused by mutations in the gene encoding alpha-L-iduronidase (IDUA), which ultimately causes toxic buildup of glycosaminoglycans (GAGs). There is currently no cure and standard treatments provide insufficient relief to the skeletal structure and central nervous system (CNS). Human memory T (Tm) cells migrate throughout the body's tissues and can persist for years, making them an attractive approach for cellular-based, systemic enzyme replacement therapy. Here, we tested genetically engineered, IDUA-expressing Tm cells as a cellular therapy in an immunodeficient mouse model of MPS I. Our results demonstrate that a single dose of engineered Tm cells leads to detectable IDUA enzyme levels in the blood for up to 22 weeks and reduced urinary GAG excretion. Furthermore, engineered Tm cells take up residence in nearly all tested tissues, producing IDUA and leading to metabolic correction of GAG levels in the heart, lung, liver, spleen, kidney, bone marrow, and the CNS, although only minimal improved cognition was observed. Our study indicates that genetically engineered Tm cells hold great promise as a platform for cellular-based enzyme replacement therapy for the treatment of mucopolysaccharidosis type I and potentially many other enzymopathies and protein deficiencies.
Keywords: CRISPR-Cas9; Hurler syndrome; gene therapy; genome engineering; homology-directed repair; lysosomal storage disorders; memory T cells; mucopolysaccharidosis type I.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests A patent has been filed by the University of Minnesota covering technologies described in this manuscript.
Figures


Update of
-
Engineering Memory T Cells as a platform for Long-Term Enzyme Replacement Therapy in Lysosomal Storage Disorders.bioRxiv [Preprint]. 2024 Apr 28:2024.04.23.590790. doi: 10.1101/2024.04.23.590790. bioRxiv. 2024. Update in: Mol Ther. 2024 Nov 6;32(11):3865-3878. doi: 10.1016/j.ymthe.2024.09.033. PMID: 38712248 Free PMC article. Updated. Preprint.
Similar articles
-
Engineering Memory T Cells as a platform for Long-Term Enzyme Replacement Therapy in Lysosomal Storage Disorders.bioRxiv [Preprint]. 2024 Apr 28:2024.04.23.590790. doi: 10.1101/2024.04.23.590790. bioRxiv. 2024. Update in: Mol Ther. 2024 Nov 6;32(11):3865-3878. doi: 10.1016/j.ymthe.2024.09.033. PMID: 38712248 Free PMC article. Updated. Preprint.
-
Enzyme replacement therapy with laronidase (Aldurazyme(®)) for treating mucopolysaccharidosis type I.Cochrane Database Syst Rev. 2016 Apr 1;4:CD009354. doi: 10.1002/14651858.CD009354.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Jun 18;6:CD009354. doi: 10.1002/14651858.CD009354.pub5. PMID: 27033167 Updated.
-
Enzyme replacement therapy with laronidase (Aldurazyme(®)) for treating mucopolysaccharidosis type I.Cochrane Database Syst Rev. 2013 Nov 21;(11):CD009354. doi: 10.1002/14651858.CD009354.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Apr 01;4:CD009354. doi: 10.1002/14651858.CD009354.pub4. PMID: 24257962 Updated.
-
Enzyme replacement therapy with laronidase (Aldurazyme) for treating mucopolysaccharidosis type I.Cochrane Database Syst Rev. 2013 Sep 26;(9):CD009354. doi: 10.1002/14651858.CD009354.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2013 Nov 21;(11):CD009354. doi: 10.1002/14651858.CD009354.pub3. PMID: 24085657 Updated.
-
Neonatal nonviral gene editing with the CRISPR/Cas9 system improves some cardiovascular, respiratory, and bone disease features of the mucopolysaccharidosis I phenotype in mice.Gene Ther. 2020 Feb;27(1-2):74-84. doi: 10.1038/s41434-019-0113-4. Epub 2019 Dec 11. Gene Ther. 2020. PMID: 31827259
References
-
- Clarke L.A. In: GeneReviews®. Adam M.P., Feldman J., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J., Gripp K.W., Amemiya A., editors. University of Washington, Seattle; 1993. Mucopolysaccharidosis Type I. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

