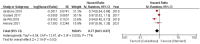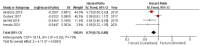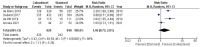The Efficacy of Cabazitaxel in Treating Prostate Cancer: A Systematic Review and Meta-Analysis
- PMID: 39367721
- PMCID: PMC11459482
- DOI: 10.1177/15579883241285162
The Efficacy of Cabazitaxel in Treating Prostate Cancer: A Systematic Review and Meta-Analysis
Abstract
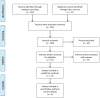
Cabazitaxel, a second-generation taxane chemotherapy agent, has demonstrated efficacy in treating metastatic castration-resistant prostate cancer (mCRPC) in patients who have previously received docetaxel-based therapy. By targeting microtubule dynamics, cabazitaxel inhibits cancer cell division and induces apoptosis, thereby extending survival and delaying disease progression in this challenging patient population. A systematic review and meta-analysis were done by searching the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE (including MEDLINE InProcess; OvidSP), Web of Science, Embase (OvidSP), and Scopus databases. ROB2 Cochrane tools assessment for RCTs. In the analysis, we used RevMan Cochrane software. Our research reveals significantly improved outcomes in terms of patient survival rates, both progression-free survival (PFS) and overall survival (OS), for cabazitaxel over comparative treatment (PFS HR 0.77 [0.61, 0.97]) (OS HR 0.79 [0.70, 0.88]). The treatment response rates were also favorable for cabazitaxel, reported as PSA Reduction Response of more than 50% (PRR) (odds ratio (OR) = 1.59 [0.56, 4.52]) and tumor response rate (TRR) (OR = 2.34 [1.28, 4.28]). Cabazitaxel was associated with significantly more incidence of adverse events. The risk ratio (RR) for serious adverse events was 1.64 [1.14, 2.35] for cabazitaxel compared to the current regimen. A systematic review and meta-analysis were done by searching in the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE (including MEDLINE InProcess; OvidSP), Web of Science, Embase (OvidSP), and Scopus databases. ROB2 Cochrane tools assessment for RCTs. In the analysis, we used RevMan Cochrane software.
Keywords: cabazitaxel; efficacy; prostate cancer.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Annala M., Fu S., Bacon J. V. W., Sipola J., Iqbal N., Ferrario C., Ong M., Wadhwa D., Hotte S. J., Lo G., Tran B., Wood L. A., Gingerich J. R., North S. A., Pezaro C. J., Ruether J. D., Sridhar S. S., Kallio H. M. L., Khalaf D. J., . . .Chi K. N. (2021). Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase II trial. Annals of Oncology, 32(7), 896–905. - PubMed
-
- Azarenko O., Smiyun G., Mah J., Wilson L., Jordan M. A. (2014). Antiproliferative mechanism of action of the novel taxane cabazitaxel as compared with the parent compound docetaxel in MCF7 breast cancer cells. Molecular Cancer Therapeutics, 13(8), 2092–2103. - PubMed
-
- Calcagno F., Nguyen T., Dobi E., Villanueva C., Curtit E., Kim S., Montcuquet P., Kleinclauss F., Pivot X., Thiery-Vuillemin A. (2013). Safety and efficacy of cabazitaxel in the docetaxel-treated patients with hormone-refractory prostate cancer. Clinical Medicine Insights: Oncology, 7, 1–12. - PMC - PubMed
-
- de Bono J. S., Oudard S., Ozguroglu M., Hansen S., Machiels J. P., Kocak I., Gravis G., Bodrogi I., Mackenzie M. J., Shen L., Roessner M., Gupta S., Sartor A. O., & TROPIC Investigators. (2010). Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. The Lancet, 376(9747), 1147–1154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous