Matrix-assisted laser desorption/ionization mass spectrometry imaging as a new tool for molecular histopathology in epilepsy surgery
- PMID: 39367795
- PMCID: PMC11647443
- DOI: 10.1111/epi.18136
Matrix-assisted laser desorption/ionization mass spectrometry imaging as a new tool for molecular histopathology in epilepsy surgery
Abstract
Objective: Epilepsy surgery is a treatment option for patients with seizures that do not respond to pharmacotherapy. The histopathological characterization of the resected tissue has an important prognostic value to define postoperative seizure outcome in these patients. However, the diagnostic classification process based on microscopic assessment remains challenging, particularly in the case of focal cortical dysplasia (FCD). Imaging mass spectrometry is a spatial omics technique that could improve tissue phenotyping and patient stratification by investigating hundreds of biomolecules within a single tissue sample, without the need for target-specific reagents.
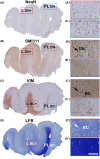
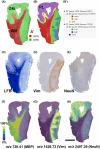
Methods: An in situ proteomic technique called matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) is here investigated as a potential new tool to expand conventional diagnosis on standard paraffin brain tissue sections. Unsupervised and region of interest-based MALDI-MSI analyses of sections from 10 FCD type IIb (FCDIIb) cases were performed, and the results were validated by immunohistochemistry.
Results: MALDI-MSI identified distinct histopathological features and the boundaries of the dysplastic lesion. The capability to visualize the spatial distribution of well-known diagnostic markers enabling multiplex measurements on single tissue sections was demonstrated. Finally, a fingerprint list of potential discriminant peptides that distinguish FCD core from peri-FCD tissue was generated.
Significance: This is the first study that explores the potential application of MALDI-MSI in epilepsy postsurgery fixed tissue, by utilizing the well-characterized FCDIIb features as a model. Extending these preliminary analyses to a larger cohort of patients will generate spectral libraries of molecular signatures that discriminate tissue features and will contribute to patient phenotyping.
Keywords: FFPE; epilepsy surgery; focal cortical dysplasia; human tissues; mass spectrometry imaging; peptides.
© 2024 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
The authors declare no conflict of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



Similar articles
-
Clinical, imaging, and immunohistochemical characteristics of focal cortical dysplasia Type II extratemporal epilepsies in children: analyses of an institutional case series.J Neurosurg Pediatr. 2017 Feb;19(2):182-195. doi: 10.3171/2016.8.PEDS1686. Epub 2016 Nov 25. J Neurosurg Pediatr. 2017. PMID: 27885945
-
Epilepsy surgery for focal cortical dysplasia: Seizure and quality of life (QOLIE-89) outcomes.Neurol India. 2018 Nov-Dec;66(6):1655-1666. doi: 10.4103/0028-3886.246263. Neurol India. 2018. PMID: 30504559
-
A deep-learning-based histopathology classifier for focal cortical dysplasia (FCD) unravels a complex scenario of comorbid FCD subtypes.Epilepsia. 2024 Dec;65(12):3501-3512. doi: 10.1111/epi.18161. Epub 2024 Oct 23. Epilepsia. 2024. PMID: 39440630 Free PMC article.
-
Recent advances in matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI-MSI) for in situ analysis of endogenous molecules in plants.Phytochem Anal. 2018 Jul;29(4):351-364. doi: 10.1002/pca.2759. Epub 2018 Apr 17. Phytochem Anal. 2018. PMID: 29667236 Review.
-
MALDI mass spectrometry imaging: A cutting-edge tool for fundamental and clinical histopathology.Proteomics Clin Appl. 2016 Jul;10(7):701-19. doi: 10.1002/prca.201500140. Epub 2016 Jun 16. Proteomics Clin Appl. 2016. PMID: 27188927 Review.
Cited by
-
Mini Review: Highlight of Recent Advances and Applications of MALDI Mass Spectrometry Imaging in 2024.Anal Sci Adv. 2025 May 10;6(1):e70016. doi: 10.1002/ansa.70016. eCollection 2025 Jun. Anal Sci Adv. 2025. PMID: 40352425 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

