Determination of double bond positions in unsaturated fatty acids by pre-column derivatization with dimethyl and dipyridyl disulfide followed by LC-SWATH-MS analysis
- PMID: 39367908
- PMCID: PMC12052876
- DOI: 10.1007/s00216-024-05542-z
Determination of double bond positions in unsaturated fatty acids by pre-column derivatization with dimethyl and dipyridyl disulfide followed by LC-SWATH-MS analysis
Abstract
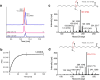
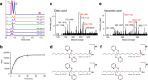
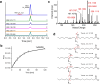
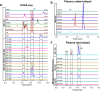
Comprehensive in-depth structural characterization of free mono-unsaturated and polyunsaturated fatty acids often requires the determination of carbon-carbon double bond positions due to their impact on physiological properties and relevance in biological samples or during impurity profiling of pharmaceuticals. In this research, we report on the evaluation of disulfides as suitable derivatization reagents for the determination of carbon-carbon double bond positions of unsaturated free fatty acids by UHPLC-ESI-QTOF-MS/MS analysis and SWATH (sequential windowed acquisition of all theoretical mass spectra) acquisition. Iodine-catalyzed derivatization of C = C double bonds with dimethyl disulfide (DMDS) enabled detection of characteristic carboxy-terminal MS2 fragments for various fatty acids in ESI negative mode. The determination of double bond positions of fatty acids with up to three double bonds, the transfer of the method to plasma samples, and its limitations have been shown. To achieve charge-switching for positive ion mode MS-detection, derivatization with 2,2'-dipyridyldisulfide (DPDS) was investigated. It enabled detection of both corresponding characteristic omega-end- and carboxy-end-fragments for fatty acids with up to two double bonds in positive ion mode. It provides a straightforward strategy for designing MRM transitions for targeted LC-MS/MS assays. Both derivatization techniques represent a simple and inexpensive way for the determination of double bond positions in fatty acids with low number of double bonds. No adaptation of MS hardware is required and the specific isotopic pattern of resulting sulfur-containing products provides additional structural confirmation. This reaction scheme opens up the avenue of structural tuning of disulfide reagents beyond DMDS and DPDS using reagents like cystine and analogs to achieve enhanced performance and sensitivity.
Keywords: 2,2′-Dipyridyldisulfide (DPDS); Collision-induced dissociation (CID); Data-independent acquisition (DIA); Dimethyl disulfide (DMDS); Isomer; Lipidomics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures

References
-
- Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006;1(4):420–39. 10.1002/biot.200600012. - PubMed
-
- Olfert M, Bäurer S, Wolter M, Buckenmaier S, Brito-de la Fuente E, Lämmerhofer M. Comprehensive profiling of conjugated fatty acid isomers and their lipid oxidation products by two-dimensional chiral RP×RP liquid chromatography hyphenated to UV- and SWATH-MS-detection. Anal Chim Acta. 2022;1202:339667. 10.1016/j.aca.2022.339667. - PubMed
-
- Simopoulos AP, Bazán NG, editors. Omega-3 fatty acids, the brain and retina. Karger Medical and Scientific Publishers; 2009.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

