Apache is a neuronal player in autophagy required for retrograde axonal transport of autophagosomes
- PMID: 39367928
- PMCID: PMC11455771
- DOI: 10.1007/s00018-024-05441-7
Apache is a neuronal player in autophagy required for retrograde axonal transport of autophagosomes
Erratum in
-
Correction: Apache is a neuronal player in autophagy required for retrograde axonal transport of autophagosomes.Cell Mol Life Sci. 2025 Jul 30;82(1):295. doi: 10.1007/s00018-025-05675-z. Cell Mol Life Sci. 2025. PMID: 40736704 Free PMC article. No abstract available.
Abstract
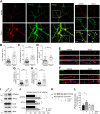
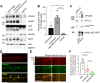
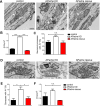
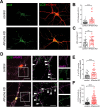
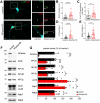
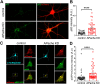
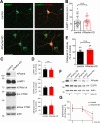
Neurons are dependent on efficient quality control mechanisms to maintain cellular homeostasis and function due to their polarization and long-life span. Autophagy is a lysosomal degradative pathway that provides nutrients during starvation and recycles damaged and/or aged proteins and organelles. In neurons, autophagosomes constitutively form in distal axons and at synapses and are trafficked retrogradely to the cell soma to fuse with lysosomes for cargo degradation. How the neuronal autophagy pathway is organized and controlled remains poorly understood. Several presynaptic endocytic proteins have been shown to regulate both synaptic vesicle recycling and autophagy. Here, by combining electron, fluorescence, and live imaging microscopy with biochemical analysis, we show that the neuron-specific protein APache, a presynaptic AP-2 interactor, functions in neurons as an important player in the autophagy process, regulating the retrograde transport of autophagosomes. We found that APache colocalizes and co-traffics with autophagosomes in primary cortical neurons and that induction of autophagy by mTOR inhibition increases LC3 and APache protein levels at synaptic boutons. APache silencing causes a blockade of autophagic flux preventing the clearance of p62/SQSTM1, leading to a severe accumulation of autophagosomes and amphisomes at synaptic terminals and along neurites due to defective retrograde transport of TrkB-containing signaling amphisomes along the axons. Together, our data identify APache as a regulator of the autophagic cycle, potentially in cooperation with AP-2, and hypothesize that its dysfunctions contribute to the early synaptic impairments in neurodegenerative conditions associated with impaired autophagy.
Keywords: AP-2; Amphisome; LC3; Retrograde trafficking; Synapse; Torin1; TrkB; mTOR.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

