Circulating tumor DNA predicts recurrence and survival in patients with resectable gastric and gastroesophageal junction cancer
- PMID: 39369091
- PMCID: PMC11706848
- DOI: 10.1007/s10120-024-01556-9
Circulating tumor DNA predicts recurrence and survival in patients with resectable gastric and gastroesophageal junction cancer
Abstract
Background: Gastric and gastroesophageal junction (GEJ) cancer represents a significant global health challenge, with high recurrence rates and poor survival outcomes. This study investigates circulating tumor DNA (ctDNA) as a biomarker for assessing recurrence risk in patients with resectable gastric and GEJ adenocarcinomas (AC).
Methods: Patients with resectable gastric and GEJ AC, undergoing perioperative chemotherapy and surgery, were prospectively enrolled. Serial plasma samples were collected at baseline, after one cycle of chemotherapy, after preoperative chemotherapy, and after surgery. ctDNA was assessed by a ddPCR test (TriMeth), which targets the gastrointestinal cancer-specific methylation patterns of the genes C9orf50, KCNQ5, and CLIP4.
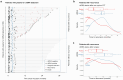
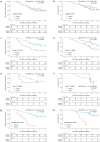
Results: ctDNA analysis was performed on 229 plasma samples from 86 patients. At baseline, ctDNA was detected in 56% of patients, which decreased to 37% following one cycle of chemotherapy, 25% after preoperative chemotherapy and 15% after surgical resection. The presence of ctDNA after one cycle of chemotherapy was associated with reduced recurrence-free survival (RFS) (HR = 2.54, 95% confidence interval (CI) 1.33-4.85, p = 0.005) and overall survival (OS) (HR = 2.23, 95% CI 1.07-4.62, p = 0.032). Similarly, ctDNA after surgery was associated with significantly shorter RFS (HR = 6.22, 95% CI 2.39-16.2, p < 0.001) and OS (HR = 6.37, 95% CI 2.10-19.3, p = 0.001). Multivariable regression analysis confirmed ctDNA after surgery as an independent prognostic factor (p < 0.001).
Conclusion: ctDNA analysis has the potential to identify patients at elevated risk of recurrence, thus providing personalized treatment strategies for patients with resectable gastric and GEJ cancer. Further validation in larger cohorts and ctDNA-guided interventions are needed for future clinical use.
Keywords: Circulating tumor DNA; Curative treatment; DNA methylation; Gastroesophageal cancer; Tumor biomarkers.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflict of interest. Ethical approval and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. This study was performed in line with the principles of the Declaration of Helsinki. Approvals were granted from The Ethics Committee of the Capital Region Denmark for the collection and use of biological samples and the capture of clinical data from patient journals. Informed consent was obtained from all participating patients. The Danish Data Protection Agency approved the study.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- GLOBOCAN [Internet]. Lyon: World Health Organization, International Agency for Research on Cancer; c2024 [cited 2024 Feb 10]. Available from: https://gco.iarc.fr/
-
- Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):992–1004. - PubMed
-
- Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20. - PubMed
MeSH terms
Substances
Grants and funding
- R320-A18521/Kræftens Bekæmpelse
- R257-A14700/Kræftens Bekæmpelse
- DKF-2021-104 - (704)/Dansk Kræftforsknings Fond
- 20220926_3/DCCC ctDNA Research Center - The Danish Research Center for Circulating Tumor DNA Guided Cancer Management
- 200414-10/DCCC ctDNA Research Center - The Danish Research Center for Circulating Tumor DNA Guided Cancer Management
LinkOut - more resources
Full Text Sources
Medical

