AP-1B regulates interactions of epithelial cells and intraepithelial lymphocytes in the intestine
- PMID: 39369131
- PMCID: PMC11455912
- DOI: 10.1007/s00018-024-05455-1
AP-1B regulates interactions of epithelial cells and intraepithelial lymphocytes in the intestine
Abstract
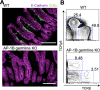
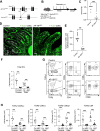
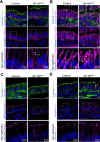
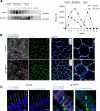
Intraepithelial lymphocytes (IELs) reside in the epithelial layer and protect against foreign pathogens, maintaining the epithelial barrier function in the intestine. Interactions between IEL and epithelial cells are required for IELs to function effectively; however, the underlying molecular machinery remains to be elucidated. In this study, we found that intestinal epithelium-specific deficiency of the clathrin adaptor protein (AP)-1B, which regulates basolateral protein sorting, led to a massive reduction in IELs. Quantitative proteomics demonstrated that dozens of proteins, including known IEL-interacting proteins (E-cadherin, butyrophilin-like 2, and plexin B2), were decreased in the basolateral membrane of AP-1B-deficient epithelial cells. Among these proteins, CD166 interacted with CD6 on the surface of induced IEL. CD166 knockdown, using shRNA in intestinal organoid cultures, significantly inhibited IEL recruitment to the epithelial layer. These findings highlight the essential role of AP-1B-mediated basolateral sorting in IEL maintenance and survival within the epithelial layer. This study reveals a novel function of AP-1B in the intestinal immune system.
Keywords: Alcam; Induced IEL; Iodixanol density gradient ultracentrifugation; Natural IEL; TMT-based quantitative proteome analysis.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures






References
-
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269:1872–1875. 10.1126/science.7569928 - PubMed
-
- Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS (1999) μ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett 449(2–3):215–220. 10.1016/s0014-5793(99)00432-9 - PubMed
-
- Fölsch H, Ohno H, Bonifacino JS, Mellman I (1999) A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99:189–198. 10.1016/s0092-8674(00)81650-5 - PubMed
MeSH terms
Substances
Grants and funding
- 19K07239/Japan Society for the Promotion of Science
- 23H02739/Japan Society for the Promotion of Science
- 20H05876/Japan Society for the Promotion of Science
- 20H00509/Japan Society for the Promotion of Science
- 22K19445/Japan Society for the Promotion of Science
- 23H05482/Japan Society for the Promotion of Science
- JPMJPR19H3/Core Research for Evolutional Science and Technology
- JPMJCR19H3/Core Research for Evolutional Science and Technology
- JPMJCR19H1/Core Research for Evolutional Science and Technology
- JPMJMS2025/Moonshot Research and Development Program
- 22gm1310009h0003/Japan Agency for Medical Research and Development
- Program for the Advancement of Next Generation Research Projects/Keio University
- Doctorate Student Grant-in-Aid Program from Ushioda Memorial Fund/Keio University
- FY2022/Asahi Glass Foundation
- FY2023/Asahi Glass Foundation
- FY2022/Secom Science and Technology Foundation
- FY2023/Fuji Foundation for Protein Research
- FY2024/Fuji Foundation for Protein Research
- Kibou Projects/Japanese Society for Immunology (JP)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

