Plasma and CSF neurofilament light chain distinguish neurodegenerative from primary psychiatric conditions in a clinical setting
- PMID: 39369278
- PMCID: PMC11567869
- DOI: 10.1002/alz.14278
Plasma and CSF neurofilament light chain distinguish neurodegenerative from primary psychiatric conditions in a clinical setting
Abstract
Introduction: People with neurodegenerative disorders (ND) frequently face diagnostic delay and misdiagnosis. We investigated blood and cerebrospinal fluid (CSF) neurofilament light chain (NfL) to distinguish ND from primary psychiatric disorders (PPD), a common challenge in clinical settings.
Methods: Plasma and CSF NfL levels were measured and compared between groups, adjusting for age, sex, and weight.
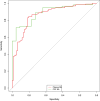
Results: A total of 337 participants were included: 136 ND, 77 PPD, and 124 Controls. Plasma NfL was 2.5-fold elevated in ND compared to PPD and had strong diagnostic performance (area under the curve, [AUC]: 0.86, 81%/85% specificity/sensitivity) that was comparable to CSF NfL (2-fold elevated, AUC: 0.89, 95%/71% specificity/sensitivity). Diagnostic performance was especially strong in younger people (40- < 60 years). Additional findings were cutoffs optimized for sensitivity and specificity, and issues important for future clinical translation.
Conclusions: This study adds important evidence for a simple blood-based biomarker to assist as a screening test for neurodegeneration and distinction from PPD, in clinical settings.
Highlights: NfL levels were significantly higher in ND versus PPD. Plasma NfL showed strong diagnostic performance, comparable to CSF NfL, to distinguish ND from PPD. Diagnostic performance was higher in younger people, where diagnostic challenges are greater. Further research is needed on analytical and reference range factors, for clinical translation. These findings support a simple screening blood test for neurodegeneration.
Keywords: biomarkers; dementia; diagnosis; neurofilament light chain protein; psychiatric disorders.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). S.L. has received an honorarium from Lundbeck and previously received NHMRC funding. T.K. served on scientific advisory boards for MS International Federation and World Health Organisation, BMS, Roche, Janssen, Sanofi Genzyme, Novartis, Merck, and Biogen, the steering committee for Brain Atrophy Initiative by Sanofi Genzyme received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Roche, Sanofi‐Genzyme, Teva, BioCSL and Merck and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck. K.B. has served as a consultant and on advisory boards for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, and Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. K.B. is supported by the Swedish Research Council (#2017‐00915 and #2022‐00732), the Swedish Alzheimer Foundation (#AF‐930351, #AF‐939721, #AF‐968270, and #AF‐994551), Hjärnfonden, Sweden (#FO2017‐0243 and #ALZ2022‐0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF‐agreement (#ALFGBG‐715986 and #ALFGBG‐965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019‐466‐236), the Alzheimer's Association 2021 Zenith Award (ZEN‐21‐848495), the Alzheimer's Association 2022‐2025 Grant (SG‐23‐1038904 QC), La Fondation Recherche Alzheimer (FRA), Paris, France, the Kirsten and Freddy Johansen Foundation, Copenhagen, Denmark, and Familjen Rönströms Stiftelse, Stockholm, Sweden. This study was supported by funding from NHMRC (1185180), MACH MRFF RART 2.2, CJDSGN Memorial Award in memory of Michael Luscombe and RMH Foundation Spring Appeal. All the other authors have nothing to disclose. Author disclosures are available in the Supporting Information.
Figures

References
-
- Eratne D, Loi SM, Walia N, et al. A pilot study of the utility of cerebrospinal fluid neurofilament light chain in differentiating neurodegenerative from psychiatric disorders: a “C‐reactive protein” for psychiatrists and neurologists?. Aust N Z J Psychiatry. 2020;54:57‐67. doi:10.1177/0004867419857811 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

