Brexpiprazole treatment for agitation in Alzheimer's dementia: A randomized study
- PMID: 39369280
- PMCID: PMC11567808
- DOI: 10.1002/alz.14282
Brexpiprazole treatment for agitation in Alzheimer's dementia: A randomized study
Abstract
Introduction: We evaluated the efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer's dementia (AAD) in Japanese patients.
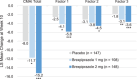
Methods: This was a phase 2/3 multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Patients with AAD were randomized to receive brexpiprazole 1 mg/day or 2 mg/day, or placebo (3:4:4) for 10 weeks.
Results: For the primary endpoint (change in Cohen-Mansfield Agitation Inventory [CMAI] total score from baseline to Week 10), both brexpiprazole 1 mg and 2 mg groups demonstrated statistically significant improvement versus placebo (2 mg: least squares [LS] mean difference -7.2 [95% confidence interval (CI): -10.0 to -4.3], p-value < 0.0001, 1 mg: LS mean difference -3.7 [95% CI: -6.8 to -0.7], p-value = 0.0175). The incidences of treatment-emergent adverse events reported in the brexpiprazole 1 mg, 2 mg, and placebo groups were 76.8%, 84.6%, and 73.8%, respectively.
Discussion: Brexpiprazole 1 mg/day and 2 mg/day for 10 weeks was efficacious and well tolerated.
Highlights: Brexpiprazole treatment for 10 weeks improved agitation in Alzheimer's dementia. The efficacy of brexpiprazole 1 mg/day has been confirmed for the first time. The incidence of adverse events was higher compared to the previous studies. Both brexpiprazole 1 mg/day and 2 mg/day were generally well tolerated.
Keywords: Alzheimer's disease; Japan; brexpiprazole; efficacy; safety.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Yu Nakamura has received speakers’ honoraria, manuscript fee, research support, or scholarship donation from Otsuka Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Viatris Pharmaceutical K.K., Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., Teikoku Pharmaceutical K.K., Kowa Company Ltd., Mochida Pharmaceutical Co., Ltd., Towa Pharmaceutical Co., Ltd, MSD K.K., Biogen Japan Ltd., and Daiichi Sankyo Company Ltd. Jun Adachi, Naoki Hirota, Katsuhiro Iba, Koichi Shimizu, Masami Nakai, Kaneyoshi Takahashi, and Naoki Mori are full‐time employees of Otsuka Pharmaceutical Co., Ltd. Author disclosures are available in the Supporting Information.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

