GLUT1 overexpression in CAR-T cells induces metabolic reprogramming and enhances potency
- PMID: 39370422
- PMCID: PMC11456602
- DOI: 10.1038/s41467-024-52666-y
GLUT1 overexpression in CAR-T cells induces metabolic reprogramming and enhances potency
Abstract
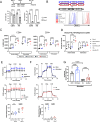
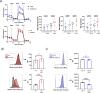
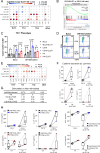
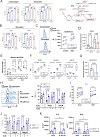
The intensive nutrient requirements needed to sustain T cell activation and proliferation, combined with competition for nutrients within the tumor microenvironment, raise the prospect that glucose availability may limit CAR-T cell function. Here, we seek to test the hypothesis that stable overexpression (OE) of the glucose transporter GLUT1 in primary human CAR-T cells would improve their function and antitumor potency. We observe that GLUT1OE in CAR-T cells increases glucose consumption, glycolysis, glycolytic reserve, and oxidative phosphorylation, and these effects are associated with decreased T cell exhaustion and increased Th17 differentiation. GLUT1OE also induces broad metabolic reprogramming associated with increased glutathione-mediated resistance to reactive oxygen species, and increased inosine accumulation. When challenged with tumors, GLUT1OE CAR-T cells secrete more proinflammatory cytokines and show enhanced cytotoxicity in vitro, and demonstrate superior tumor control and persistence in mouse models. Our collective findings support a paradigm wherein glucose availability is rate limiting for effector CAR-T cell function and demonstrate that enhancing glucose availability via GLUT1OE could augment antitumor immune function.
© 2024. The Author(s).
Conflict of interest statement
C.L.M. is a cofounder of and holds equity in Link Cell Therapies, CARGO therapeutics and GBM NewCo. E.S holds equity in Lyell Immunopharma and consults for Lepton Pharmaceuticals and Galaria. S.A.F. serves on the Scientific Advisory Boards for Alaunos Therapeutics and Fresh Wind Biotech and has equity interest in both; S.A.F. receives research funding from CARGO and Tune Therapeutics. C.L.M. receives research funding from Lyell, Tune and consults for CARGO, Link, GBMNewCo, Immatics, and Ensoma. K.L.D is a St. Baldrick’s Foundation Scholar and acknowledges support from Hyundai Hope on Wheels and Oxnard Foundation (K.L.D.). K.C. is currently employed by AstraZeneca. The remaining authors declare no competing interests.
Figures


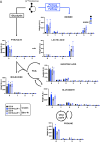
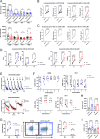
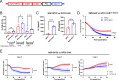
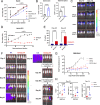
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- R01 CA263500/CA/NCI NIH HHS/United States
- R01 DK125260/DK/NIDDK NIH HHS/United States
- R01DK125260/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30DK116074/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

