Phytotherapeutic BS012 and Its Active Component Ameliorate Allergic Asthma via Inhibition of Th2-Mediated Immune Response and Apoptosis
- PMID: 39370723
- PMCID: PMC11535288
- DOI: 10.4062/biomolther.2024.058
Phytotherapeutic BS012 and Its Active Component Ameliorate Allergic Asthma via Inhibition of Th2-Mediated Immune Response and Apoptosis
Abstract
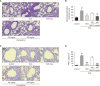
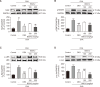
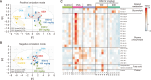
Asthma is a chronic inflammatory disorder of the lungs that results in airway inflammation and narrowing. BS012 is an herbal remedy containing Asarum sieboldii, Platycodon grandiflorum, and Cinnamomum cassia extracts. To elucidate the anti-asthma effect of BS012, this study analyzed the immune response, respiratory protection, and changes in metabolic mechanisms in an ovalbumin-induced allergic asthma mouse model. Female BALB/c mice were exposed to ovalbumin to induce allergic asthma. Bronchoalveolar lavage fluid and plasma were analyzed for interleukin and immunoglobulin E levels. Histological analyses of the lungs were performed to measure morphological changes. Apoptosis-related mediators were assayed by western blotting. Plasma and lung tissue metabolomic analyses were performed to investigate the metabolic changes. A T-helper-2-like differentiated cell model was used to identify the active components of BS012. BS012 treatment improved inflammatory cell infiltration, mucus production, and goblet cell hyperplasia in lung tissues. BS012 also significantly downregulated ovalbumin-specific immunoglobulin E in plasma and T-helper-2-specific cytokines, interleukin-4 and -5, in bronchoalveolar lavage fluid. The lungs of ovalbumin-inhaled mice exhibited nerve growth factor-mediated apoptotic protein expression, which was significantly attenuated by BS012 treatment. Ovalbumin-induced abnormalities in amino acid and lipid metabolism were improved by BS012 in correlation with its anti-inflammatory properties and normalization of energy metabolism. Additionally, the differentiated cell model revealed that N-isobutyl-dodecatetraenamide is an active component that contributes to the anti-allergic properties of BS012. The current findings demonstrate the anti-allergic and respiratory protective functions of BS012 against allergic asthma, which can be considered a therapeutic candidate.
Keywords: Apoptosis; Asthma; Inflammation; Metabolomics; Ovalbumin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Cottrill K. A., Chandler J. D., Kobara S., Stephenson S. T., Mohammad A. F., Tidwell M., Mason C., Van Dresser M., Patrignani J., Kamaleswaran R., Fitzpatrick A. M., Grunwell J. R. Metabolomics identifies disturbances in arginine, phenylalanine, and glycine metabolism as differentiating features of exacerbating atopic asthma in children. J. Allergy Clin. Immunol. Glob. 2023;2:100115. doi: 10.1016/j.jacig.2023.100115. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

