Causal association between metabolites and upper gastrointestinal tumors: A Mendelian randomization study
- PMID: 39370813
- PMCID: PMC11450430
- DOI: 10.3892/mmr.2024.13336
Causal association between metabolites and upper gastrointestinal tumors: A Mendelian randomization study
Abstract
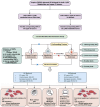
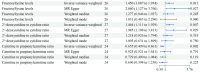
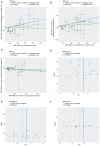
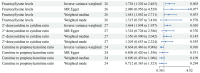
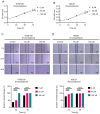
Upper gastrointestinal (UGI) tumors, notably gastric cancer (GC) and esophageal cancer (EC), are significant global health concerns due to their high morbidity and mortality rates. However, only a limited number of metabolites have been identified as biomarkers for these cancers. To explore the association between metabolites and UGI tumors, the present study conducted a comprehensive two‑sample Mendelian randomization (MR) analysis using publicly available genetic data. In the present study, the causal relationships were examined between 1,400 metabolites and UGI cancer using methods such as inverse variance weighting and weighted medians, along with sensitivity analyses for heterogeneity and pleiotropy. Functional experiments were conducted to validate the MR results. The analysis identified 57 metabolites associated with EC and 58 with GC. Key metabolites included fructosyllysine [EC: Odds ratio (OR)=1.450, 95% confidence interval (CI)=1.087‑1.934, P=0.011; GC: OR=1.728, 95% CI=1.202‑2.483, P=0.003], 2'‑deoxyuridine to cytidine ratio (EC: OR=1.464, 95% CI=1.111‑1.929, P=0.007; GC: OR=1.464, 95% CI=1.094‑1.957, P=0.010) and carnitine to protonylcarnitine (C3) ratio (EC: OR=0.655, 95% CI=0.499‑0.861, P=0.002; GC: OR=0.664, 95% CI=0.486‑0.906, P=0.010). Notably, fructosyllysine levels and the 2'‑deoxyuridine to cytidine ratio were identified as risk factors for both EC and GC, while the C3 ratio served as a protective factor. Functional experiments demonstrated that fructosyllysine and the 2'‑deoxyuridine to cytidine ratio promoted the proliferation of EC and GC cells, whereas carnitine inhibited their proliferation. In conclusion, the present findings provide insights into the causal factors and biomarkers associated with UGI tumors, which may be instrumental in guiding targeted dietary and pharmacological interventions, thereby contributing to the prevention and treatment of UGI cancer.
Keywords: Mendelian randomization; UK Biobank; esophageal cancer; gastric cancer; metabolites.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

