The epithelial barrier theory and its associated diseases
- PMID: 39370939
- PMCID: PMC11657050
- DOI: 10.1111/all.16318
The epithelial barrier theory and its associated diseases
Abstract
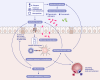
The prevalence of many chronic noncommunicable diseases has been steadily rising over the past six decades. During this time, over 350,000 new chemical substances have been introduced to the lives of humans. In recent years, the epithelial barrier theory came to light explaining the growing prevalence and exacerbations of these diseases worldwide. It attributes their onset to a functionally impaired epithelial barrier triggered by the toxicity of the exposed substances, associated with microbial dysbiosis, immune system activation, and inflammation. Diseases encompassed by the epithelial barrier theory share common features such as an increased prevalence after the 1960s or 2000s that cannot (solely) be accounted for by the emergence of improved diagnostic methods. Other common traits include epithelial barrier defects, microbial dysbiosis with loss of commensals and colonization of opportunistic pathogens, and circulating inflammatory cells and cytokines. In addition, practically unrelated diseases that fulfill these criteria have started to emerge as multimorbidities during the last decades. Here, we provide a comprehensive overview of diseases encompassed by the epithelial barrier theory and discuss evidence and similarities for their epidemiology, genetic susceptibility, epithelial barrier dysfunction, microbial dysbiosis, and tissue inflammation.
Keywords: epidemiology; epithelial barrier; epithelial barrier dysfunction; inflammation; microbial dysbiosis.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
M.S. has received research grants from the Swiss National Science Foundation (SNSF no.: 310030_189334/1), GSK, Novartis, Stiftung vorm. Bündner Heilstätte Arosa and OM Pharma as well as speaker's fee from AstraZeneca. W.V. has received research grants from PROMEDICA Stiftung, Switzerland, and EoE Stiftung, Switzerland, and consulting fees form Mabylon AG, Switzerland. M.C.B. reports grants from the Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, Leo Foundation, LEO Pharmacy, and Freenovation, advisory board fees from AstraZeneca, Almirall, LEO Pharma, lecture honorarium from Almirall and AstraZeneca, and patient education grant from AstraZeneca and GSK. A.S. has currently consultant contracts with Astra‐Seneca, BMS‐Receptos, Calypso, EsoCap, Falk‐Pharma, GSK, and Sanofi‐Regeneron. E.G.Y. is an employee of Mount Sinai and has received research grants from and/or is a consultant for: Research Grants (paid to the institution): Boehringer Ingelheim, Leo Pharma, Pfizer, Cara Therapeutics, UCB, Kyowa Kirin, RAPT, Amgen, GSK, Incyte, Sanofi, Bristol Meyers Squibb, Aslan, Regeneron, Anaptysbio, Concert, Janssen. Consultant: Abbvie, Aclaris, Almirall, Amgen, AnaptysBio, Apogee Therapeutics, Apollo Therapeutics Limited, Artax Biopharma Inc., AstraZeneca, Bristol Meyers Squibb, Boerhinger‐Ingelhiem, Cara Therapeutics, Centrexion Therapeutics Corporation, Connect Biopharm, DBV TECHNOLOGIES, Eli Lilly, Enveda Biosciences, Escient Pharmaceuticals, Inc., Fairmount Funds Management LLC, Forest Laboratories Galderma, Gate Bio, Google Ventures (GV), GSK Immunology, Horizon Therapeutics USA, Inc., Incyte, Inmagene, Janssen Biotech, JT Central Pharmaceutical Research Institute, Jasper Therapeutics, Kyowa Kirin, Leo Pharma, Merck, Nektar Therapeutics, Novartis Pharmaceuticals Corporation, NUMAB Therapeutics AG, OrbiMed Advisors LLC, OTSUKA, Pfizer, Pharmaxis Ltd, Pioneering Medicine VII, Inc., Proteologix US Inc, RAPT, Regeneron Pharmaceuticals, RibonTherapeutics, Inc., Sanofi, SATO, Schrödinger Inc., Sun Pharma Advanced Research Company (SPARC), Teva Branded Pharmaceutical Products R&D, UCB. A.F.S. reports grants from the Medical Research Council (MR/M008517/1; MC/PC/18052; MR/T032081/1), Food Allergy Research and Education (FARE), the Immune Tolerance Network/National Institute of Allergy and Infectious Diseases (NIAID, NIH), Asthma UK (AUK‐BC‐2015‐01), BBSRC, Rosetrees Trust and the NIHR through the Biomedical Research Centre (BRC) award to Guy's and St Thomas' NHS Foundation Trust, during the conduct of the study; personal fees from Thermo Scientific, Nestle, Novartis, Allergy Therapeutics, IgGenix, Buhlmann, as well as research support from IgGenix, Buhlmann, and Thermo Fisher Scientific through a collaboration agreement with King's College London. S.D.G. reports speaker fees from AstraZeneca, Chiesi, GSK, Novartis, Sanofi, Stallergenes and Takeda; advisory board fees from AstraZeneca, Chiesi, CSL‐Behring, GSK, Novartis, Sanofi, Takeda; unrestricted research grants from AstraZeneca, CSL‐Behring, GSK, Novartis, and Sanofi. O.P. reports grants and/or personal fees and/or travel support from ALK‐Abell
Figures


References
-
- Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739‐751. - PubMed
-
- D'Amato G, Akdis CA. Desert dust and respiratory diseases: further insights into the epithelial barrier hypothesis. Allergy. 2022;77:3490‐3492. - PubMed
-
- Akdis CA. The epithelial barrier hypothesis proposes a comprehensive understanding of the origins of allergic and other chronic noncommunicable diseases. J Allergy Clin Immunol. 2022;149(1):41‐44. - PubMed
-
- Sözener ZC, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145(6):1517‐1528. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

