Direct Organocatalytic Chemoselective Synthesis of Pharmaceutically Active 1,2,3-Triazoles and 4,5'-Bitriazoles
- PMID: 39371323
- PMCID: PMC11450731
- DOI: 10.1021/acsorginorgau.4c00032
Direct Organocatalytic Chemoselective Synthesis of Pharmaceutically Active 1,2,3-Triazoles and 4,5'-Bitriazoles
Abstract
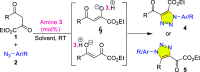
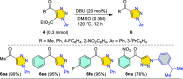
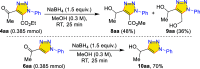
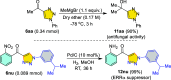
Carbonyl-containing 1,4,5-trisubstituted- and 1,4-disubstituted-1,2,3-triazoles are well-known for their wide range of applications in pharmaceutical and medicinal chemistry, but their high-yielding metal-free synthesis has always remained challenging, as no comprehensive protocol has been outlined to date. Owing to their structural and medicinal importance, herein, we synthesized various carbonyl-containing 1,4,5-trisubstituted- and 1,4-disubstituted-1,2,3-triazoles and unsymmetrical 4,5'-bitriazoles with high yields and chemo-/regioselectivity from the library of 2,4-diketoesters and azides in a sequential one-pot manner through the combination of organocatalytic enolization, in situ [3 + 2]-cycloaddition, and hydrolysis reactions. The commercial availability of the starting materials/catalysts, diverse substrate scope, performance in a one-pot manner, chemo-/regioselectivity of organo-click reaction, quick synthesis of unsymmetrical 4,5'-bitriazoles, a large number of synthetic applications, and numerous medicinal applications of carbonyl-containing 1,2,3-triazoles are the key attractions of this metal-free organo-click work.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


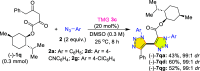
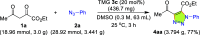
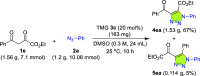


Similar articles
-
Direct organocatalytic chemoselective synthesis of pharmaceutically active benzothiazole/benzoxazole-triazoles.Org Biomol Chem. 2025 Feb 26;23(9):2142-2152. doi: 10.1039/d4ob01527d. Org Biomol Chem. 2025. PMID: 39849920
-
Enamine/enolate-mediated organocatalytic azide-carbonyl [3+2] cycloaddition reactions for the synthesis of densely functionalized 1,2,3-triazoles.Angew Chem Int Ed Engl. 2014 Dec 22;53(52):14310-2. doi: 10.1002/anie.201409410. Epub 2014 Nov 17. Angew Chem Int Ed Engl. 2014. PMID: 25404559
-
An organocatalytic azide-aldehyde [3+2] cycloaddition: high-yielding regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles.Angew Chem Int Ed Engl. 2014 Sep 22;53(39):10420-4. doi: 10.1002/anie.201406721. Epub 2014 Jul 30. Angew Chem Int Ed Engl. 2014. PMID: 25079606
-
Recent Progress on Synthesis of Functionalized 1,5-Disubstituted Triazoles.Curr Org Synth. 2024;21(4):513-558. doi: 10.2174/1570179420666230418123350. Curr Org Synth. 2024. PMID: 38804327 Review.
-
Computational studies on the regioselectivity of metal-catalyzed synthesis of 1,2,3 triazoles via click reaction: a review.J Mol Model. 2015 Oct;21(10):264. doi: 10.1007/s00894-015-2810-2. Epub 2015 Sep 18. J Mol Model. 2015. Retraction in: J Mol Model. 2019 Mar 7;25(4):86. doi: 10.1007/s00894-019-3978-7. PMID: 26385849 Retracted. Review.
References
LinkOut - more resources
Full Text Sources
