Improving Palliative Care Research Reporting: A Guide to Reporting Guidelines
- PMID: 39371500
- PMCID: PMC11450853
- DOI: 10.25259/IJPC_61_2024
Improving Palliative Care Research Reporting: A Guide to Reporting Guidelines
Abstract
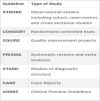
Improving the quality of research reporting is crucial for addressing current challenges in palliative care, with academic journals playing a crucial role in promoting clear and comprehensive reporting through structured guidelines. These guidelines, such as Appraisal of Guidelines, Research, and evaluation, Consolidated Standards of Reporting Trials, Case Reports (CARE) guidelines, transparent reporting of evaluations with nonrandomized designs (TREND), transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD), meta-analysis of observational studies in epidemiology (MOOSE) Checklist, methods of researching end-of-life care Statement, Preferred Reporting Items for Systematic Reviews and Meta-analyses, Standards for Quality Improvement Reporting Excellence 2.0, Strengthening the Reporting of Observational Studies in Epidemiology, standard protocol items: recommendations for interventional trials (SPIRIT), template for intervention description and replication (TIDieR) Consolidated Criteria for Reporting Qualitative Research and Standards for Qualitative Research, are instrumental in ensuring transparency by furnishing essential details for comprehending, replicating and applying research findings in clinical decision-making and systematic reviews. The Enhancing the quality and transparency of health research (EQUATOR) network champions trustworthy health research literature globally by advocating for transparent and accurate reporting, thereby enhancing the reliability and utility of research outcomes research outcomes.
Keywords: Palliative care; Reporting guidelines; Research reporting.
© 2024 Published by Scientific Scholar on behalf of Indian Journal of Palliative Care.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources