A rapid method to assess the in vivo multi-functionality of adoptively transferred engineered TCR T cells
- PMID: 39371522
- PMCID: PMC11452736
- DOI: 10.1093/immadv/ltae007
A rapid method to assess the in vivo multi-functionality of adoptively transferred engineered TCR T cells
Abstract
Introduction: The clinical efficacy of chimeric antigen and T cell receptor (TCR) T cell immunotherapies is attributed to their ability to proliferate and persist in vivo. Since the interaction of the engineered T cells with the targeted tumour or its environment might suppress their function, their functionality should be characterized not only before but also after adoptive transfer.
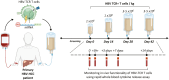
Materials and methods: We sought to achieve this by adapting a recently developed Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapid whole blood T cell assay to stimulate engineered TCR T cells in small volumes of whole blood (<1 ml) without in vitro cellular purification. As a proof-of-concept, we used this method to longitudinally study two patients with primary Hepatitis B Virus (HBV)-related hepatocellular carcinoma who received multiple dose-escalating infusions of transiently functional mRNA-engineered HBV-TCR T cells.
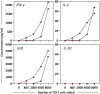
Results: We demonstrated that a simple pulsing of whole blood with a peptide corresponding to the epitope recognized by the specific HBV-TCR elicited Th1 cytokine secretion in both patients only after HBV-TCR T cell treatment and not before. The amount of cytokines secreted also showed an infusion-dose-dependent association.
Discussions: These findings support the utility of the whole blood cytokine release assay in monitoring the in vivo function and quantity of engineered T cell products following adoptive transfer.
Keywords: T cell therapy; adoptive cell transfer; hepatitis B; hepatocellular carcinoma.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors disclose the following: A.B. and A.T.T. are the Scientific Founder and the Scientific Consultant of Lion TCR Pte. Ltd. respectively, a biotech company developing T cell receptors for treatment of virus-related diseases and cancers. R.W.W and L-E.W. are employees of Lion TCR Pte. Ltd. All other authors disclose no conflicts. Lion TCR Pte. Ltd. has a patent application related to this work (WO2021148110A1).
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
