Metabolomic characterization of human glioblastomas and patient plasma: a pilot study
- PMID: 39371551
- PMCID: PMC11452765
- DOI: 10.12688/f1000research.143642.5
Metabolomic characterization of human glioblastomas and patient plasma: a pilot study
Abstract
Background: Glioblastoma (GBM) is a clinically challenging primary brain tumor with poor survival outcome despite surgical resection and intensive chemoradiation. The metabolic heterogeneity of GBM can become biomarkers for treatment response, resistance, and outcome prediction. The aim of the study is to investigate metabolic distinctions between primary and recurrent GBM tissue and patient plasma to establish feasibility for metabolic profiling.
Methods: A single-center cohort study analyzed tissue and blood samples from 15 patients with GBM using untargeted metabolomic/lipidomic assays. Metabolomic, lipidomic, and biogenic amine analyses were conducted on GBM tissue and patient plasma at diagnosis and recurrence using untargeted mass spectrometry. The study utilized a small but longitudinally collected cohort to evaluate alteration in metabolites, lipids, and biogenic amines between specimens at diagnosis and recurrence.
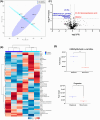
Results: Exploratory analysis revealed significant alteration in metabolites, lipids, and biogenic amines between diagnostic and recurrent states in both tumor and plasma specimens. Notable metabolites differed at recurrence, including N-alpha-methylhistamine, glycerol-3-phosphate, phosphocholine, and succinic acid in tissue, and indole-3-acetate, and urea in plasma. Principal component analysis revealed distinct metabolomic profiles between tumor tissue and patient plasma. Distinct metabolic profiles were observed in GBM tissue and patient plasma at recurrence, demonstrating the feasibility of using metabolomic methodologies for longitudinal studies. One patient exhibited a unique tumor resistance signature at diagnosis, possibly indicating a high-risk metabolomic phenotype.
Conclusions: In this small cohort, the findings suggest the potential of metabolomic signatures of GBM tissue and patient plasma for risk stratification, outcome prediction, and the development of novel adjuvant metabolic-targeting therapies. The findings suggest metabolic discrepancies at diagnosis and recurrence in tissue and plasma, highlighting potential implications for evaluation of clinical response. The identification of significant changes in metabolite abundance emphasizes the need for larger studies using targeted metabolomics to validate and further explore these profiles.
Keywords: biomarker; feasibility; glioblastoma; pilot; untargeted metabolomics.
Copyright: © 2024 Liu YA et al.
Conflict of interest statement
No competing interests were disclosed.
Figures



Update of
-
A pilot study on metabolomic characterization of human glioblastomas and patient plasma.Res Sq [Preprint]. 2023 Mar 10:rs.3.rs-2662020. doi: 10.21203/rs.3.rs-2662020/v1. Res Sq. 2023. Update in: F1000Res. 2024 Sep 19;13:98. doi: 10.12688/f1000research.143642.5. PMID: 36945517 Free PMC article. Updated. Preprint.
References
-
- Hanahan D: Hallmarks of Cancer: New Dimensions. Cancer Discov. Jan 2022;12(1):31–46. 10.1158/2159-8290.CD-21-1059 - DOI - PubMed

