Intracellular TAS2Rs act as a gatekeeper for the excretion of harmful substances via ABCB1 in keratinocytes
- PMID: 39372126
- PMCID: PMC11452442
- DOI: 10.1096/fba.2024-00074
Intracellular TAS2Rs act as a gatekeeper for the excretion of harmful substances via ABCB1 in keratinocytes
Abstract
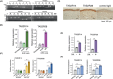
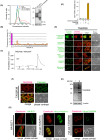
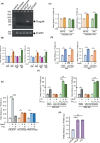
Bitter taste receptors (TAS2Rs) are not only expressed in the oral cavity but also in skin. Extraoral TAS2Rs are thought to be involved in non-taste perception and tissue-specific functions. Keratinocytes that express TAS2Rs in the skin provide a first-line defense against external threats. However, the functional roles of these receptors in host defense remain unclear. Here, we demonstrated the sensory role of intracellularly located TAS2Rs against toxic substances in keratinocytes. Although many G protein-coupled receptors elicit signals from the surface, TAS2Rs were found to localize intracellularly, possibly to the ER, in human keratinocytes and HaCaT cells. TAS2R38, one of the TAS2R members, activated the Gα12/13/RhoA/ROCK/p38 MAP kinase/NF-κB pathway upon stimulation by phenylthiocarbamide (PTC), an agonist for this receptor, leading to the production of ABC transporters, such as ABCB1, in these cells. Notably, treatment with bitter compounds, such as PTC and saccharin, induced the upregulation of ABCB1 in HaCaT cells. Mechanistically, intracellular TAS2R38 and its downstream signaling Gα12/13/RhoA/ROCK/p38 MAP kinase/NF-κB pathway were identified to be responsible for the above effect. Pretreatment with PTC prevented the accumulation of rhodamine 123 because of its excretion via ABCB1. Furthermore, pretreatment with PTC or saccharin counteracted the effect of the toxic compound, diphenhydramine, and pretreated HaCaT cells were found to proliferate faster than untreated cells. This anti-toxic effect was suppressed by treatment with verapamil, an ABCB1 inhibitor, indicating that enhanced ABCB1 helps clear toxic substances. Altogether, harmless activators of TAS2Rs may be promising drugs that enhance the excretion of toxic substances from the human skin.
Keywords: ABCB1; bitter taste receptors; host defense; intracellular location; keratinocytes: GPCR.
©2024 The Authors FASEB BioAdvances published by The Federation of American Societies for Experimental Biology.
Figures




References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693‐702. - PubMed
-
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225‐229. - PubMed
-
- Meyerhof W, Batram C, Kuhn C, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157‐170. - PubMed
LinkOut - more resources
Full Text Sources
