Entorhinal cortex-hippocampal circuit connectivity in health and disease
- PMID: 39372192
- PMCID: PMC11449717
- DOI: 10.3389/fnhum.2024.1448791
Entorhinal cortex-hippocampal circuit connectivity in health and disease
Abstract
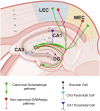
The entorhinal cortex (EC) and hippocampal (HC) connectivity is the main source of episodic memory formation and consolidation. The entorhinal-hippocampal (EC-HC) connection is classified as canonically glutamatergic and, more recently, has been characterized as a non-canonical GABAergic connection. Recent evidence shows that both EC and HC receive inputs from dopaminergic, cholinergic, and noradrenergic projections that modulate the mnemonic processes linked to the encoding and consolidation of memories. In the present review, we address the latest findings on the EC-HC connectivity and the role of neuromodulations during the mnemonic mechanisms of encoding and consolidation of memories and highlight the value of the cross-species approach to unravel the underlying cellular mechanisms known. Furthermore, we discuss how EC-HC connectivity early neurodegeneration may contribute to the dysfunction of episodic memories observed in aging and Alzheimer's disease (AD). Finally, we described how exercise may be a fundamental tool to prevent or decrease neurodegeneration.
Keywords: Alzheimer’s disease; aging; entorhinal cortex; episodic memory; exercise; hippocampus; neuromodulation.
Copyright © 2024 Hernández-Frausto and Vivar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Ahnaou A., Moechars D., Raeymaekers L., Biermans R., Manyakov N. V., Bottelbergs A., et al. (2017). Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci. Rep. 7, 14189–14114. doi: 10.1038/s41598-017-13839-6, PMID: - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

