Evaluation of the beneficial effects of a GABA-based product containing Melissa officinalis on post-inflammatory irritable bowel syndrome: a preclinical study
- PMID: 39372212
- PMCID: PMC11449869
- DOI: 10.3389/fphar.2024.1466824
Evaluation of the beneficial effects of a GABA-based product containing Melissa officinalis on post-inflammatory irritable bowel syndrome: a preclinical study
Abstract
Introduction: Visceral pain represents the most common digestive issue, frequently resulting from long-term inflammation, such as inflammatory bowel diseases. The lack of effective drugs prompted search of new therapeutic approaches. In this regard, gamma-aminobutyric acid (GABA) and Melissa officinalis (Mo) appear as excellent candidates as they were recognized to have several positive effects on the digestive system. The aim of this research was to evaluate the effects of a compound containing GABA and Mo (GABA-Mo 5:1) in inflammation-induced intestinal damage and visceral pain.
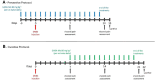
Methods: Colitis was induced in rats by intrarectal 2,4-dinitrobenzenesulfonic acid (DNBS) administration. DNBS-treated animals received GABA-Mo (80 mg/kg BID), starting 3 days before DNBS administration, until 14 days after colitis induction (preventive protocol), or starting 7 days after DNBS until day 21 (curative protocol). Visceral pain was assessed by measuring the viscero-motor response (VMR) and the abdominal withdrawal reflex (AWR) to colorectal distension on day 7, 14 (both protocols) and 21 (curative protocol) after DNBS administration.
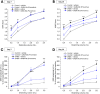
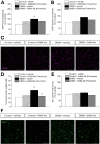
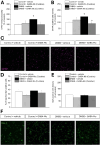
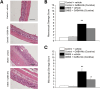
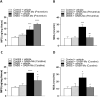
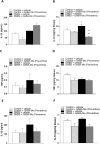
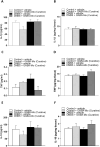
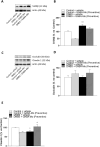
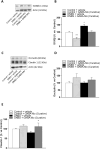
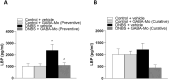
Results: In the preventive protocol, GABA-Mo reduced AWR at day 14 but had no effect on VMR. In the spinal cord, treatment with GABA-Mo significantly prevented microglia reactivity (Iba-1 positive cells). In the colon, the supplement significantly decreased malondialdehyde (MDA, index of oxidative stress) and IL-1β levels and counteracted the decreased expression of claudin-1. Moreover, GABA-Mo normalized the increased levels of plasma lipopolysaccharide binding protein (LBP, index of altered intestinal permeability). In the curative protocol, GABA-Mo significantly counteracted visceral hypersensitivity persistence in DNBS-treated animals (day 14 and 21). In the spinal cord, GABA-Mo significantly reduced GFAP positive cell density (astrocytes). Histological evaluations highlighted a mild but significant effect of GABA-Mo in promoting healing from DNBS-induced colon damage. Colonic MDA and myeloperoxidase (index of leukocyte infiltration) levels were reduced, while the decreased colonic claudin-1 expression was normalized. In addition, the increased levels of plasma LBP were normalized by GABA-Mo administration.
Discussion: In conclusion GABA-Mo, particularly in the curative protocol, was able to reduce visceral pain and intestinal inflammation, likely through a reinforcement of intestinal barrier integrity, thus representing a suitable approach for the management of abdominal pain, especially in the remission stages of colitis.
Keywords: GABA; Melissa officinalis; abdominal pain; colitis; irritable bowel syndrome.
Copyright © 2024 Lucarini, Benvenuti, Di Salvo, D’Antongiovanni, Pellegrini, Valdiserra, Ciampi, Antonioli, Lambiase, Cancelli, Grosso, Di Cesare Mannelli, Bellini, Ghelardini and Fornai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Abd Allah H. N., Abdul-Hamid M., Mahmoud A. M., Abdel-Reheim E. S. (2022). Melissa officinalis L. ameliorates oxidative stress and inflammation and upregulates Nrf2/HO-1 signaling in the hippocampus of pilocarpine-induced rats. Environ. Sci. Pollut. Res. Int. 29, 2214–2226. 10.1007/s11356-021-15825-y - DOI - PubMed
-
- Antonioli L., Pellegrini C., Fornai M., Benvenuti L., D'Antongiovanni V., Colucci R., et al. (2021). Preclinical development of FA5, a novel AMP-activated protein kinase (AMPK) activator as an innovative drug for the management of bowel inflammation. Int. J. Mol. Sci. 22, 6325. 10.3390/ijms22126325 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

