Epigenetic regulation in epithelial cells and innate lymphocyte responses to S. Typhi infection: insights into IFN-γ production and intestinal immunity
- PMID: 39372404
- PMCID: PMC11450450
- DOI: 10.3389/fimmu.2024.1448717
Epigenetic regulation in epithelial cells and innate lymphocyte responses to S. Typhi infection: insights into IFN-γ production and intestinal immunity
Abstract
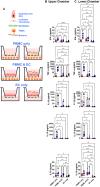
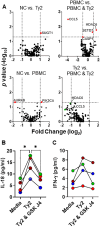
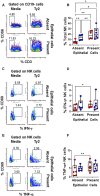
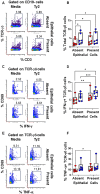
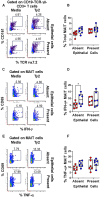
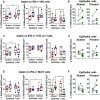
Infection by Salmonella enterica serovar Typhi (S. Typhi), the cause of enteric fevers, is low in high-income countries but persistent in low- and middle-income countries, resulting in 65,400-187,700 deaths yearly. Drug resistance, including in the United States, exacerbates this issue. Evidence indicates that innate lymphocytes (INLs), such as natural killer (NK) cells, and unconventional T lymphocytes (e.g., Mucosal-associated invariant T (MAIT) cells and T-cell receptor gamma delta (TCR-γδ) cells) can impact the intestinal epithelial barrier, the primary site of exposure to S. Typhi. Moreover, INL production of IFN-γ is central in controlling S. Typhi infection. However, the impact of epithelial cells (EC) on the secretion of IFN-γ by INLs and the relationship between these events and epigenetic changes remains unknown. Epigenetic modifications in host cells are fundamental for their differentiation and function, including IFN-γ production. Herein, using a human organoid-derived polarized intestinal epithelial cell monolayer, we investigated the role of H3K4me3 and H3K27me3 epigenetic marks in intestinal immunity, focusing on the function of EC, NK, MAIT, and TCR-γδ cells in response to S. Typhi. This study builds on our previous findings that MAIT subsets exhibiting specific IFN-γ pattern signatures were associated with protection against typhoid fever and that S. Typhi infection regulates changes in chromatin marks that depend on individual cell subsets. Here, we show that cultures exposed to S. Typhi without EC exhibit a significant increase in NK and MAIT cells, and, to a lesser extent, TCR-γδ cells, expressing IFN-γ and H3K4me3 but not H3K27me3 marks, contrasting with cultures where EC is present. The influence of EC on INL H3K4me3 marks might be indirectly mediated through the modulation of IL-18 secretion via the Histone Deacetylase 6 gene during S. Typhi infection.
Keywords: bacteria; epigenetic; gut; human; salmonella.
Copyright © 2024 Salerno-Goncalves, Chen, Bafford and Sztein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Figures
References
-
- Typhoid fever and paratyphoid fever, centers for disease control and prevention. (2024). Available at: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/typhoid....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

