Tumor-associated lymphatic vessel density is a reliable biomarker for prognosis of esophageal cancer after radical resection: a systemic review and meta-analysis
- PMID: 39372418
- PMCID: PMC11449706
- DOI: 10.3389/fimmu.2024.1453482
Tumor-associated lymphatic vessel density is a reliable biomarker for prognosis of esophageal cancer after radical resection: a systemic review and meta-analysis
Abstract
Purpose: To explore whether tumor-associated lymphatic vessel density (LVD) could be a biomarker for the prognosis of patients with esophageal cancer after radical resection.
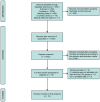
Methods: A systematic literature search was performed through PubMed, EMBASE, Wanfang Data, and Cochrane Library from the inception of databases until March 19, 2024. The selected studies investigated overall survival (OS) and/or recurrence-free survival (RFS) of patients with esophageal cancer with different levels of LVD after radical resection. The OS and RFS data were pooled as hazard ratios (HR) and 95% confidential interval (CI). Furthermore, the standardized mean differences (SMDs) and 95% CI were aggregated to evaluate the correlation between LVD and clinicopathological features.
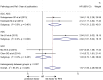
Results: A total of 10 retrospective studies of 1,201 patients were finally included for the meta-analysis. Patients with esophageal cancer with a high level of LVD exhibited worse OS (HR 1.65, 95% CI 1.18 to 2.31) and RFS (HR 1.57, 95% CI 1.09 to 2.26) than those with a low level of LVD. Subgroup analysis of different pathological subtypes revealed that patients with esophageal adenocarcinoma with a high level of LVD had significantly worse RFS (HR 2.84, 95% CI 1.61 to 5.02) than those with a low level of LVD; while patients with esophageal squamous cell carcinoma with a high level of LVD had similar OS (HR 1.52, 95% CI 0.93 to 2.47) and RFS (HR 1.03, 95% CI 0.72 to 1.48) to those with a low level of LVD. Furthermore, tumors with lymph node metastasis had significantly higher levels of LVD than those without lymph node metastasis (SMD = 1.11, 95% CI 0.54 to 1.67). Tumors at the stages III-IV had significantly higher levels of LVD than those at the stages I-II (SMD = 1.62, 95% CI 0.90 to 2.34).
Conclusion: A high level of LVD in tumor was associated with worse survival of patients with esophageal cancer after radical resection, especially in patients with esophageal adenocarcinoma. Tumor-associated LVD is a new parameter that should be measured in postoperative pathology for predicting the prognosis of patients with esophageal cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero/ PROSPERO, identifier CRD42024553766.
Keywords: esophageal cancer; lymphangiogenesis; lymphatic vessel density; meta-analysis; overall survival; prognosis; radical resection; recurrence-free survival.
Copyright © 2024 Li, Wang, Liang, Chen, Luo, Li, Chen, Lu and Ke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

