This is a preprint.
Lineage-specific CDK activity dynamics characterize early mammalian development
- PMID: 39372752
- PMCID: PMC11451597
- DOI: 10.1101/2024.06.14.599098
Lineage-specific CDK activity dynamics characterize early mammalian development
Update in
-
Lineage-specific CDK activity dynamics characterize early mammalian development.Cell Rep. 2025 Apr 22;44(4):115558. doi: 10.1016/j.celrep.2025.115558. Epub 2025 Apr 11. Cell Rep. 2025. PMID: 40220290 Free PMC article.
Abstract
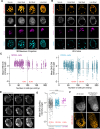
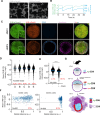
Cyclin-dependent kinases (CDK) are key regulatory enzymes that regulate proliferation dynamics and cell fate in response to extracellular inputs. It remains largely unknown how CDK activity fluctuates and influences cell commitment in vivo during early mammalian development. Here, we generated a transgenic mouse model expressing a CDK kinase translocation reporter (KTR) that enabled quantification of CDK activity in live single cells. By examining pre- and post-implantation mouse embryos at different stages, we observed a progressive decrease in CDK activity in cells from the trophectoderm (TE) prior to implantation. This drop correlated with the establishment of an FGF4-dependent signaling gradient through the embryonic-abembryonic axis. Furthermore, we showed that CDK activity levels do not determine cell fate decisions during pre-implantation development. Finally, we uncovered the existence of conserved regulatory mechanisms in mammals by revealing lineage-specific regulation of CDK activity in TE-like human cells.
Keywords: CDK; Embryonic stem cells; Kinase translocation reporter; Pre-implantation development; Trophectoderm.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures


References
-
- Aleem E., Kiyokawa H. and Kaldis P. (2005) Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7, 831–836. - PubMed
-
- Bedzhov I., Leung C.Y., Bialecka M. and Zernicka-Goetz M. (2014) In vitro culture of mouse blastocysts beyond the implantation stages. Nat. Protoc. 9, 2732–2739. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
