This is a preprint.
Genomic analyses identify 15 susceptibility loci and reveal HDAC2, SOX2-OT, and IGF2BP2 in a naturally-occurring canine model of gastric cancer
- PMID: 39372775
- PMCID: PMC11451740
- DOI: 10.1101/2024.08.14.604426
Genomic analyses identify 15 susceptibility loci and reveal HDAC2, SOX2-OT, and IGF2BP2 in a naturally-occurring canine model of gastric cancer
Update in
-
Genomic analyses identify 15 risk loci and reveal HDAC2, SOX2-OT, and IGF2BP2 in a naturally occurring canine model of gastric cancer.Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2416723122. doi: 10.1073/pnas.2416723122. Epub 2025 May 30. Proc Natl Acad Sci U S A. 2025. PMID: 40445765 Free PMC article.
Abstract
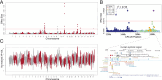
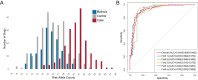
Gastric cancer (GC) is the fifth most common human cancer worldwide, but the genetic etiology is largely unknown. We performed a Bayesian genome-wide association study and selection analyses in a naturally-occurring canine model of GC, the Belgian Tervuren and Sheepdog breeds, to elucidate underlying genetic risk factors. We identified 15 loci with over 90% predictive accuracy for the GC phenotype. Variant filtering revealed germline putative regulatory variants for the EPAS1 (HIF2A) and PTEN genes and a coding variant in CD101. Although closely related to Tervuren and Sheepdogs, Belgian Malinois rarely develop GC. Across-breed analyses uncovered protective haplotypes under selection in Malinois at SOX2-OT and IGF2BP2. Among Tervuren and Sheepdogs, HDAC2 putative regulatory variants were present at comparatively high frequency and were associated with GC. Here, we describe a complex genetic architecture governing GC in a dog model, including genes such as PDZRN3, that have not been associated with human GC.
Keywords: Bayesian GWAS; Belgian Sheepdog; Groenendael; Malinois; PDZRN3; Tervuren; adenocarcinoma; canine; dog; selection.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures


References
-
- Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., et al. (2018). Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet 391, 1023–1075. 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
-
- Arnold M., Rutherford M.J., Bardot A., Ferlay J., Andersson T.M.-L., Myklebust T.Å., Tervonen H., Thursfield V., Ransom D., Shack L., et al. (2019). Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 20, 1493–1505. 10.1016/S1470-2045(19)30456-5. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
