This is a preprint.
Cryo-electron tomography pipeline for plasma membranes
- PMID: 39372776
- PMCID: PMC11451596
- DOI: 10.1101/2024.06.27.600657
Cryo-electron tomography pipeline for plasma membranes
Update in
-
Cryo-electron tomography pipeline for plasma membranes.Nat Commun. 2025 Jan 20;16(1):855. doi: 10.1038/s41467-025-56045-z. Nat Commun. 2025. PMID: 39833141 Free PMC article.
Abstract
Cryo-electron tomography (cryoET) provides sub-nanometer protein structure within the dense cellular environment. Existing sample preparation methods are insufficient at accessing the plasma membrane and its associated proteins. Here, we present a correlative cryo-electron tomography pipeline optimally suited to image large ultra-thin areas of isolated basal and apical plasma membranes. The pipeline allows for angstrom-scale structure determination with sub-tomogram averaging and employs a genetically-encodable rapid chemically-induced electron microscopy visible tag for marking specific proteins within the complex cell environment. The pipeline provides fast, efficient, distributable, low-cost sample preparation and enables targeted structural studies of identified proteins at the plasma membrane of cells.
Figures
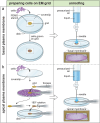

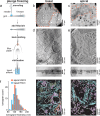



References
-
- Bäuerlein F.J. and Baumeister W., Towards visual proteomics at high resolution. Journal of Molecular Biology, 2021. 433(20): p. 167187. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
