This is a preprint.
α-Synuclein Conformations in Plasma Distinguish Parkinson's Disease from Dementia with Lewy Bodies
- PMID: 39372921
- PMCID: PMC11451739
- DOI: 10.21203/rs.3.rs-5033901/v1
α-Synuclein Conformations in Plasma Distinguish Parkinson's Disease from Dementia with Lewy Bodies
Abstract
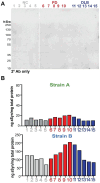
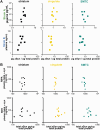
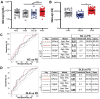
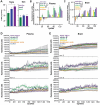
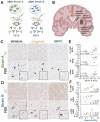
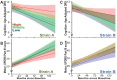
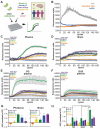
Aggregation of misfolded α-synuclein (aSyn) within the brain is the pathologic hallmark of Lewy body diseases (LBD), including Parkinson's disease (PD) and dementia with Lewy bodies (DLB). Evidence exists for aSyn "strains" - conformations with distinct biological properties. However, biomarkers for PD vs. DLB, including potential aSyn strain differences, are lacking. Here, we used two monoclonal antibodies selective for different in vitro aSyn species - termed Strain A and B - to evaluate human brain tissue, cerebrospinal fluid (CSF), and plasma. Surprisingly, levels of Strain A and B aSyn species differed in plasma from individuals with PD vs. DLB in two independent cohorts. Lower plasma aSyn Strain A species also predicted subsequent PD cognitive decline. Strain A and Strain B aSyn species were undetectable in CSF, but plasma aSyn species could template aSyn fibrillization, particularly in PD. Our findings suggest that aSyn strains may impact LBD clinical presentation and originate outside the brain.
Conflict of interest statement
Competing interests: Dr Tropea has received consulting fees and honoraria from Sanofi Genzyme, Bial, and the Parkinson Foundation. The following authors declare that they have no competing interests: GTK, SA, RSA JFM, ACP, DJI, RTS, RDR, KD, VL.
Figures








References
-
- Hogan D. B., Fiest K. M., Roberts J. I., Maxwell C. J., Dykeman J., Pringsheim T., Steeves T., Smith E. E., Pearson D., Jetté N., The Prevalence and Incidence of Dementia with Lewy Bodies: a Systematic Review. Canadian Journal of Neurological Sciences 43, S83–S95 (2016). - PubMed
Publication types
Grants and funding
- U24 NS095871/NS/NINDS NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- K08 NS093127/NS/NINDS NIH HHS/United States
- R01 NS115139/NS/NINDS NIH HHS/United States
- U19 AG062418/AG/NIA NIH HHS/United States
- R37 NS115139/NS/NINDS NIH HHS/United States
- P30 AG072979/AG/NIA NIH HHS/United States
- R01 NS082265/NS/NINDS NIH HHS/United States
- K23 NS114167/NS/NINDS NIH HHS/United States
- P01 AG084497/AG/NIA NIH HHS/United States
- K08 NS133286/NS/NINDS NIH HHS/United States
- T32 NS091008/NS/NINDS NIH HHS/United States
- P01 AG066597/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

