Time to response with ravulizumab, a long-acting terminal complement inhibitor, in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis
- PMID: 39373062
- PMCID: PMC11555155
- DOI: 10.1111/ene.16490
Time to response with ravulizumab, a long-acting terminal complement inhibitor, in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis
Abstract
Background and purpose: The efficacy and safety of ravulizumab, a terminal complement C5 inhibitor, in adults with anti-acetylcholine receptor antibody-positive (AChR Ab+) generalized myasthenia gravis (gMG) were demonstrated in the CHAMPION MG study (NCT03920293). This analysis aimed to characterize the latency to onset of a clinically meaningful therapeutic effect for ravulizumab.
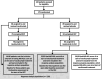
Methods: Post hoc analysis of data collected for up to 60 weeks from CHAMPION MG was performed to assess the timing of response to ravulizumab. Response was analyzed based on reductions of ≥2 and ≥3 points (minimal clinically important differences [MCIDs]) in Myasthenia Gravis-Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) total scores, respectively, and on more rigorous reductions of ≥3 and ≥5 points, respectively. Time to first response was assessed using the Kaplan-Meier product-limit method.
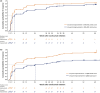
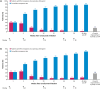
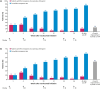
Results: The median (95% confidence interval) time to first response was 2.1 (2.1-2.6) and 4.1 (2.3-10.0) weeks for reductions of ≥2 and ≥3 points in MG-ADL total score, respectively (n = 139), and 4.1 (2.1-10.0) and 18.3 (11.0-33.4) weeks for reductions of ≥3 and ≥5 points in QMG total score, respectively (n = 134). Cumulative response rates at Week 60 (data cut-off) were 88% and 82% for ≥2- and ≥3-point MG-ADL score reductions, respectively, and 86% and 59% for ≥3- and ≥5-point QMG score reductions, respectively.
Conclusions: The median times to MCID with ravulizumab treatment in patients with AChR Ab+ gMG were ~2 weeks and ~4 weeks based on MCID MG-ADL and QMG total score reductions, respectively.
Keywords: activities of daily living; complement inactivating agents; muscle strength; myasthenia gravis, generalized.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

