CYLD Maintains Retinal Homeostasis by Deubiquitinating ENKD1 and Promoting the Phagocytosis of Photoreceptor Outer Segments
- PMID: 39373352
- PMCID: PMC11615780
- DOI: 10.1002/advs.202404067
CYLD Maintains Retinal Homeostasis by Deubiquitinating ENKD1 and Promoting the Phagocytosis of Photoreceptor Outer Segments
Abstract
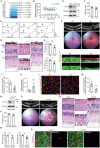
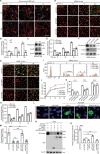
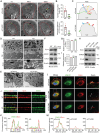
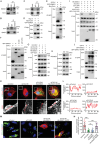
Phagocytosis of shed photoreceptor outer segments by the retinal pigment epithelium (RPE) is essential for retinal homeostasis. Dysregulation of the phagocytotic process is associated with irreversible retinal degenerative diseases. However, the molecular mechanisms underlying the phagocytic activity of RPE cells remain elusive. In an effort to uncover proteins orchestrating retinal function, the cylindromatosis (CYLD) deubiquitinase is identified as a critical regulator of photoreceptor outer segment phagocytosis. CYLD-deficient mice exhibit abnormal retinal structure and function. Mechanistically, CYLD interacts with enkurin domain containing protein 1 (ENKD1) and deubiquitinates ENKD1 at lysine residues K141 and K242. Deubiquitinated ENKD1 interacts with Ezrin, a membrane-cytoskeleton linker, and stimulates the microvillar localization of Ezrin, which is essential for the phagocytic activity of RPE cells. These findings thus reveal a crucial role for the CYLD-ENKD1-Ezrin axis in regulating retinal homeostasis and may have important implications for the prevention and treatment of retinal degenerative diseases.
Keywords: CYLD; ENKD1; deubiquitination; phagocytosis; photoreceptor; retinal pigment epithelium.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Diurnal Photoreceptor Outer Segment Renewal in Mice Is Independent of Galectin-3.Invest Ophthalmol Vis Sci. 2021 Feb 1;62(2):7. doi: 10.1167/iovs.62.2.7. Invest Ophthalmol Vis Sci. 2021. PMID: 33538769 Free PMC article.
-
Cell Culture Analysis of the Phagocytosis of Photoreceptor Outer Segments by Primary Mouse RPE Cells.Methods Mol Biol. 2018;1753:63-71. doi: 10.1007/978-1-4939-7720-8_4. Methods Mol Biol. 2018. PMID: 29564781 Free PMC article.
-
Semaphorin4D-PlexinB1 Signaling Attenuates Photoreceptor Outer Segment Phagocytosis by Reducing Rac1 Activity of RPE Cells.Mol Neurobiol. 2018 May;55(5):4320-4332. doi: 10.1007/s12035-017-0649-5. Epub 2017 Jun 17. Mol Neurobiol. 2018. PMID: 28624895 Free PMC article.
-
Role of RPE Phagocytosis in the Retina Metabolic Ecosystem.Adv Exp Med Biol. 2025;1468:429-433. doi: 10.1007/978-3-031-76550-6_70. Adv Exp Med Biol. 2025. PMID: 39930233 Review.
-
Blockade of MerTK Activation by AMPK Inhibits RPE Cell Phagocytosis.Adv Exp Med Biol. 2016;854:773-8. doi: 10.1007/978-3-319-17121-0_103. Adv Exp Med Biol. 2016. PMID: 26427488 Review.
Cited by
-
Post-Translational Modifications in Cilia and Ciliopathies.Adv Sci (Weinh). 2025 Aug;12(31):e16562. doi: 10.1002/advs.202416562. Epub 2025 May 28. Adv Sci (Weinh). 2025. PMID: 40433930 Free PMC article. Review.
-
UFMylation Modulates OFIP Stability and Centrosomal Localization.J Clin Lab Anal. 2025 Mar;39(6):e70004. doi: 10.1002/jcla.70004. Epub 2025 Mar 10. J Clin Lab Anal. 2025. PMID: 40059580 Free PMC article.
References
-
- a) Holmes D., Nature 2018, 561, S2; - PubMed
- b) Yang S., Zhou J., Li D., Front. Pharmacol. 2021, 12, 727870; - PMC - PubMed
- c) Ran J., Liu M., Feng J., Li H., Ma H., Song T., Cao Y., Zhou P., Wu Y., Yang Y., Yang Y., Yu F., Guo H., Zhang L., Xie S., Li D., Gao J., Zhang X., Zhu X., Zhou J., Dev. Cell 2020, 53, 287; - PubMed
- d) Ran J., Guo G., Zhang S., Zhang Y., Zhang L., Li D., Wu S., Cong Y., Wang X., Xie S., Zhao H., Liu H., Ou G., Zhu X., Zhou J., Liu M., Adv. Sci. 2024, 11, e2400569. - PMC - PubMed
-
- a) Strauss O., Physiol. Rev. 2005, 85, 845; - PubMed
- b) Bertolotti E., Neri A., Camparini M., Macaluso C., Marigo V., Prog Retin Eye Res. 2014, 42, 130; - PubMed
- c) Flores‐Bellver M., Mighty J., Aparicio‐Domingo S., Li K. V., Shi C., Zhou J., Cobb H., McGrath P., Michelis G., Lenhart P., Bilousova G., Heissel S., Rudy M. J., Coughlan C., Goodspeed A. E., Becerra S. P., Redenti S., Canto‐Soler M. V., J. Extracell Vesicles 2021, 10, e12165. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
