A Novel Superparamagnetic-Responsive Hydrogel Facilitates Disc Regeneration by Orchestrating Cell Recruitment, Proliferation, and Differentiation within Hostile Inflammatory Niche
- PMID: 39373392
- PMCID: PMC11600201
- DOI: 10.1002/advs.202408093
A Novel Superparamagnetic-Responsive Hydrogel Facilitates Disc Regeneration by Orchestrating Cell Recruitment, Proliferation, and Differentiation within Hostile Inflammatory Niche
Abstract
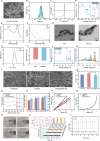
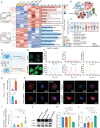
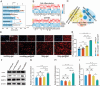
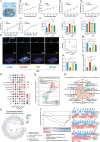
In situ disc regeneration is a meticulously orchestrated process, which involves cell recruitment, proliferation and differentiation within a local inflammatory niche. Thus far, it remains a challenge to establish a multi-staged regulatory framework for coordinating these cellular events, therefore leading to unsatisfactory outcome. This study constructs a super paramagnetically-responsive cellular gel, incorporating superparamagnetic iron oxide nanoparticles (SPIONs) and aptamer-modified palladium-hydrogen nanozymes (PdH-Apt) into a double-network polyacrylamide/hyaluronic acid (PAAm/HA) hydrogel. The Aptamer DB67 within magnetic hydrogel (Mag-gel) showed a high affinity for disialoganglioside (GD2), a specific membrane ligand of nucleus pulposus stem cells (NPSCs), to precisely recruit them to the injury site. The Mag-gel exhibits remarkable sensitivity to a magnetic field (MF), which exerts tunable micro/nano-scale forces on recruited NPSCs and triggers cytoskeletal remodeling, consequently boosting cell expansion in the early stage. By altering the parameters of MF, the mechanical cues within the hydrogel facilitates differentiation of NPSCs into nucleus pulposus cells to restore disc structure in the later stage. Furthermore, the PdH nanozymes within the Mag-gel mitigate the harsh inflammatory microenvironment, favoring cell survival and disc regeneration. This study presents a remote and multi-staged strategy for chronologically regulating endogenous stem cell fate, supporting disc regeneration without invasive procedures.
Keywords: intervertebral disc regeneration; mechanical stimulation; nucleus pulposus stem cells; superparamagnetic hydrogel.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Knezevic N. N., Candido K. D., Vlaeyen J. W. S., Van Zundert J., Cohen S. P., Lancet 2021, 398, 78. - PubMed
-
- Clouet J., Fusellier M., Camus A., Visage C. L.e, Guicheux J., Adv. Drug Delivery Rev. 2019, 146, 306. - PubMed
-
- Fine N., Lively S., Séguin C. A., Perruccio A. V., Kapoor M., Rampersaud R., Nat. Rev. Rheumatol. 2023, 19, 136. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
