Increased antibiotic resistance in preterm neonates under early antibiotic use
- PMID: 39373498
- PMCID: PMC11542550
- DOI: 10.1128/msphere.00286-24
Increased antibiotic resistance in preterm neonates under early antibiotic use
Abstract
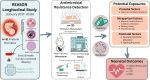
The standard use of antibiotics in newborns to empirically treat early-onset sepsis can adversely affect the neonatal gut microbiome, with potential long-term health impacts. Research into the escalating issue of antimicrobial resistance in preterm infants and antibiotic practices in neonatal intensive care units is limited. A deeper understanding of the effects of early antibiotic intervention on antibiotic resistance in preterm infants is crucial. This retrospective study employed metagenomic sequencing to evaluate antibiotic resistance genes (ARGs) in the meconium and subsequent stool samples of preterm infants enrolled in the Routine Early Antibiotic Use in Symptomatic Preterm Neonates study. Microbial metagenomics was conducted using a subset of fecal samples from 30 preterm infants for taxonomic profiling and ARG identification. All preterm infants exhibited ARGs, with 175 unique ARGs identified, predominantly associated with beta-lactam, tetracycline, and aminoglycoside resistance. Notably, 23% of ARGs was found in preterm infants without direct or intrapartum antibiotic exposure. Post-natal antibiotic exposure increases beta-lactam/tetracycline resistance while altering mechanisms that aid bacteria in withstanding antibiotic pressure. Microbial profiling revealed 774 bacterial species, with antibiotic-naive infants showing higher alpha diversity (P = 0.005) in their microbiota and resistome compared with treated infants, suggesting a more complex ecosystem. High ARG prevalence in preterm infants was observed irrespective of direct antibiotic exposure and intensifies with age. Prolonged membrane ruptures and maternal antibiotic use during gestation and delivery are linked to alterations in the preterm infant resistome and microbiome, which are pivotal in shaping the ARG profiles in the neonatal gut.This study is registered with ClinicalTrials.gov as NCT02784821.
Importance: A high burden of antibiotic resistance in preterm infants poses significant challenges to neonatal health. The presence of antibiotic resistance genes, along with alterations in signaling, energy production, and metabolic mechanisms, complicates treatment strategies for preterm infants, heightening the risk of ineffective therapy and exacerbating outcomes for these vulnerable neonates. Despite not receiving direct antibiotic treatment, preterm infants exhibit a concerning prevalence of antibiotic-resistant bacteria. This underscores the complex interplay of broader influences, including maternal antibiotic exposure during and beyond pregnancy and gestational complications like prolonged membrane ruptures. Urgent action, including cautious antibiotic practices and enhanced antenatal care, is imperative to protect neonatal health and counter the escalating threat of antimicrobial resistance in this vulnerable population.
Keywords: meconium; microbiome; preterm infants; resistome; stool.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization . 2014. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization
-
- Gasparrini AJ, Wang B, Sun X, Kennedy EA, Hernandez-Leyva A, Ndao IM, Tarr PI, Warner BB, Dantas G. 2019. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat Microbiol 4:2285–2297. doi:10.1038/s41564-019-0550-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
