Positive selection, genetic recombination, and intra-host evolution in novel equine coronavirus genomes and other members of the Embecovirus subgenus
- PMID: 39373506
- PMCID: PMC11542594
- DOI: 10.1128/spectrum.00867-24
Positive selection, genetic recombination, and intra-host evolution in novel equine coronavirus genomes and other members of the Embecovirus subgenus
Abstract
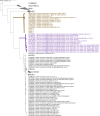
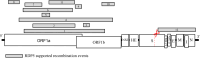
There are several examples of coronaviruses in the Betacoronavirus subgenus Embecovirus that have jumped from an animal to the human host. Studying how evolutionary factors shape coronaviruses in non-human hosts may provide insight into the coronavirus host-switching potential. Equids, such as horses and donkeys, are susceptible to equine coronaviruses (ECoVs). With increased testing prevalence, several ECoV genome sequences have become available for molecular evolutionary analyses, especially those from the United States of America (USA). To date, no analyses have been performed to characterize evolution within coding regions of the ECoV genome. Here, we obtain and describe four new ECoV genome sequences from infected equines from across the USA presenting clinical symptoms of ECoV, and infer ECoV-specific and Embecovirus-wide patterns of molecular evolution. Within two of the four data sets analyzed, we find evidence of intra-host evolution within the nucleocapsid (N) gene, suggestive of quasispecies development. We also identify 12 putative genetic recombination events within the ECoV genome, 11 of which fall in ORF1ab. Finally, we infer and compare sites subject to positive selection on the ancestral branch of each major Embecovirus member clade. Specifically, for the two currently identified human coronavirus (HCoV) embecoviruses that have spilled from animals to humans (HCoV-OC43 and HCoV-HKU1), we find that there are 42 and 2 such sites, respectively, perhaps reflective of the more complex ancestral evolutionary history of HCoV-OC43, which involves several different animal hosts.IMPORTANCEThe Betacoronavirus subgenus Embecovirus contains coronaviruses that not only pose a health threat to animals and humans, but also have jumped from animal to human host. Equids, such as horses and donkeys are susceptible to equine coronavirus (ECoV) infections. No studies have systematically examined evolutionary patterns within ECoV genomes. Our study addresses this gap and provides insight into intra-host ECoV evolution from infected horses. Further, we identify and report natural selection pattern differences between two embecoviruses that have jumped from animals to humans [human coronavirus OC43 and HKU1 (HCoV-OC43 and HCoV-HKU1, respectively)], and hypothesize that the differences observed may be due to the different animal host(s) that each virus circulated in prior to its jump into humans. Finally, we contribute four novel, high-quality ECoV genomes to the scientific community.
Keywords: coronavirus; equine coronavirus; natural selection; tropism shift.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The First Detection of Equine Coronavirus in Adult Horses and Foals in Ireland.Viruses. 2019 Oct 14;11(10):946. doi: 10.3390/v11100946. Viruses. 2019. PMID: 31615132 Free PMC article.
-
Identification of a recombinant equine coronavirus in donkey, China.Emerg Microbes Infect. 2022 Dec;11(1):1010-1013. doi: 10.1080/22221751.2022.2056522. Emerg Microbes Infect. 2022. PMID: 35311478 Free PMC article.
-
Recombination and Positive Selection Differentially Shaped the Diversity of Betacoronavirus Subgenera.Viruses. 2020 Nov 16;12(11):1313. doi: 10.3390/v12111313. Viruses. 2020. PMID: 33207802 Free PMC article.
-
Hosts and Sources of Endemic Human Coronaviruses.Adv Virus Res. 2018;100:163-188. doi: 10.1016/bs.aivir.2018.01.001. Epub 2018 Feb 16. Adv Virus Res. 2018. PMID: 29551135 Free PMC article. Review.
-
Equine Coronaviruses.Vet Clin North Am Equine Pract. 2023 Apr;39(1):55-71. doi: 10.1016/j.cveq.2022.11.008. Epub 2022 Nov 21. Vet Clin North Am Equine Pract. 2023. PMID: 36737293 Review.
References
-
- So RTY, Chu DKW, Miguel E, Perera R, Oladipo JO, Fassi-Fihri O, Aylet G, Ko RLW, Zhou Z, Cheng M-S, Kuranga SA, Roger FL, Chevalier V, Webby RJ, Woo PCY, Poon LLM, Peiris M. 2019. Diversity of dromedary camel coronavirus HKU23 in African camels revealed multiple recombination events among closely related betacoronaviruses of the subgenus embecovirus. J Virol 93:e01236-19. doi:10.1128/JVI.01236-19 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

