Target-site cefiderocol pharmacokinetics in soft tissues of healthy volunteers
- PMID: 39373642
- PMCID: PMC11638555
- DOI: 10.1093/jac/dkae359
Target-site cefiderocol pharmacokinetics in soft tissues of healthy volunteers
Abstract
Background: Cefiderocol may potentially be used to treat skin and soft tissue infections (SSTIs). However, the pharmacokinetics of cefiderocol in human soft tissues have not yet been determined. The objective of the present PK study was to investigate whether target-site concentrations of cefiderocol are sufficiently high for the treatment of SSTIs.
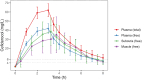
Methods: In this pharmacokinetic study, a single intravenous dose of 2 g cefiderocol was administered to eight healthy male volunteers. Drug concentrations were determined in plasma, muscle and subcutis over 8 h. Free plasma concentrations were calculated using the plasma protein binding determined with ultrafiltration. Free tissue concentrations were obtained using microdialysis. Penetration ratios were calculated as AUC0-8h_free_tissue/AUC0-8h_free_plasma. A population pharmacokinetic model was developed, and the probability of target attainment (PTA) was determined using Monte Carlo simulations.
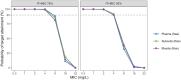
Results: Cefiderocol showed good tissue penetration, with mean penetration ratios ± standard deviation of 0.99 ± 0.33 and 0.92 ± 0.30 for subcutis and muscle, respectively. Cefiderocol pharmacokinetics in plasma were best described with a two-compartment model, and tissue concentrations were described by scaling the tissue concentrations to concentrations in the peripheral compartment of the plasma model. For a thrice-daily regimen with 2 g doses intravenously infused over 3 h, PTA was ≥90% for MIC values up to 4 mg/L, both based on free plasma and soft tissue pharmacokinetics.
Conclusions: This study indicates that a dose of 2 g cefiderocol achieves concentrations in plasma considered sufficient for treating relevant bacterial species. Assuming a comparable PK/PD target for soft tissues, sufficiently high concentrations would also be achieved in soft tissues.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

