Functional role of myosin-binding protein H in thick filaments of developing vertebrate fast-twitch skeletal muscle
- PMID: 39373654
- PMCID: PMC11461142
- DOI: 10.1085/jgp.202413604
Functional role of myosin-binding protein H in thick filaments of developing vertebrate fast-twitch skeletal muscle
Erratum in
-
Correction: Functional role of myosin-binding protein H in thick filaments of developing vertebrate fast-twitch skeletal muscle.J Gen Physiol. 2024 Dec 2;156(12):e20241360411072024c. doi: 10.1085/jgp.20241360411072024c. Epub 2024 Nov 13. J Gen Physiol. 2024. PMID: 39535407 Free PMC article. No abstract available.
Abstract
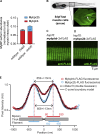
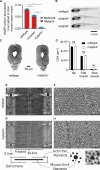
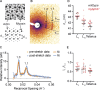
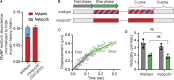
Myosin-binding protein H (MyBP-H) is a component of the vertebrate skeletal muscle sarcomere with sequence and domain homology to myosin-binding protein C (MyBP-C). Whereas skeletal muscle isoforms of MyBP-C (fMyBP-C, sMyBP-C) modulate muscle contractility via interactions with actin thin filaments and myosin motors within the muscle sarcomere "C-zone," MyBP-H has no known function. This is in part due to MyBP-H having limited expression in adult fast-twitch muscle and no known involvement in muscle disease. Quantitative proteomics reported here reveal that MyBP-H is highly expressed in prenatal rat fast-twitch muscles and larval zebrafish, suggesting a conserved role in muscle development and prompting studies to define its function. We take advantage of the genetic control of the zebrafish model and a combination of structural, functional, and biophysical techniques to interrogate the role of MyBP-H. Transgenic, FLAG-tagged MyBP-H or fMyBP-C both localize to the C-zones in larval myofibers, whereas genetic depletion of endogenous MyBP-H or fMyBP-C leads to increased accumulation of the other, suggesting competition for C-zone binding sites. Does MyBP-H modulate contractility in the C-zone? Globular domains critical to MyBP-C's modulatory functions are absent from MyBP-H, suggesting that MyBP-H may be functionally silent. However, our results suggest an active role. In vitro motility experiments indicate MyBP-H shares MyBP-C's capacity as a molecular "brake." These results provide new insights and raise questions about the role of the C-zone during muscle development.
© 2024 Mead et al.
Conflict of interest statement
Disclosures: D.M. Warshaw reported grants from Edgewise Therapeutics outside the submitted work. No other disclosures were reported.
Figures






Update of
-
Functional role of myosin-binding protein H in thick filaments of developing vertebrate fast-twitch skeletal muscle.bioRxiv [Preprint]. 2024 May 13:2024.05.10.593199. doi: 10.1101/2024.05.10.593199. bioRxiv. 2024. Update in: J Gen Physiol. 2024 Dec 2;156(12):e202413604. doi: 10.1085/jgp.202413604. PMID: 38798399 Free PMC article. Updated. Preprint.
References
-
- Adekeye, T. E., Teets E.M., Tomak E.A., Waterman S.L., Sprague K.A., White A., Coffin M.L., Varga S.M., Easterbrooks T.E., Shepherd S.J., et al. 2024. Fast-twitch myofibrils grow in proportion to Mylpf dosage in the zebrafish embryo. bioRxiv. 10.1101/2024.09.18.613721 - DOI
MeSH terms
Substances
Grants and funding
- P01 HL059408/HL/NHLBI NIH HHS/United States
- R35 GM141743/GM/NIGMS NIH HHS/United States
- S10 OD012331/OD/NIH HHS/United States
- R01 HL150953/HL/NHLBI NIH HHS/United States
- R01 AR070299/AR/NIAMS NIH HHS/United States
- S10OD025030/NH/NIH HHS/United States
- R01 AR067715/AR/NIAMS NIH HHS/United States
- R21 NS120419/NS/NINDS NIH HHS/United States
- P50 HD103525/HD/NICHD NIH HHS/United States
- P30 GM133893/GM/NIGMS NIH HHS/United States
- DE-SC0012704/U.S. Department of Energy
- S10 OD025030/OD/NIH HHS/United States
- KP1605010/DOE Office of Biological and Environmental Research
- R01 HL157487/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

