Performance of ultrasound-guided attenuation parameter and 2D shear wave elastography in patients with metabolic dysfunction-associated steatotic liver disease
- PMID: 39373742
- PMCID: PMC11914239
- DOI: 10.1007/s00330-024-11076-w
Performance of ultrasound-guided attenuation parameter and 2D shear wave elastography in patients with metabolic dysfunction-associated steatotic liver disease
Abstract
Purpose: To assess the performance and the reproducibility of ultrasound-guided attenuation parameter (UGAP) and two-dimensional shear wave elastography (2D-SWE) in patients with biopsy-proven metabolic dysfunction-associated steatotic liver disease (MASLD).
Methods: This study included consecutive adult patients with MASLD who underwent ultrasound with UGAP, 2D-SWE and percutaneous liver biopsy. The median values of 12 consecutive UGAP measurements were acquired by two independent radiologists (R1 and R2). Hepatic steatosis was graded by liver biopsy as: (0) < 5%; (1) 5-33%; (2) > 33-66%; (3) > 66%. Areas under the curve (AUCs) were calculated to determine the diagnostic performance. Inter- and intra-observer reliability was assessed with intraclass correlation coefficient (ICC).
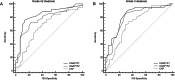
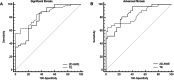
Results: A hundred patients (median age 55.0 years old) with MASLD were prospectively enrolled. At histopathology, 70 and 42 patients had grade ≥ 2 and 3 steatosis, respectively. Median UGAP was 0.78 dB/cm/MHz (IQR/Med: 5.55%). For the diagnosis of grade ≥ 2 steatosis, the AUCs of UGAP were 0.828 (95% CI: 0.739, 0.896) for R1 and 0.779 (95% CI: 0.685, 0.856) for R2. The inter- and intra-operator reliability of UGAP were excellent, with an ICC of 0.92 (95% CI: 0.87-0.95) and 0.95 (95% CI: 0.92-0.96), respectively. The median liver stiffness was 6.76 kPa (IQR/Med: 16.30%). For the diagnosis of advanced fibrosis, 2D-SWE had an AUC of 0.862 (95% CI: 0.757, 0.934), and the optimal cutoff value was > 6.75 kPa with a sensitivity of 80.6% and a specificity of 75.7%.
Conclusion: UGAP and 2D-SWE provide a good performance for the staging of steatosis and fibrosis in patients with MASLD with an excellent intra-operator reliability of UGAP.
Key points: Question How well do ultrasound-guided attenuation parameter (UGAP) and two-dimensional shear wave elastography (2D-SWE) perform for quantifying hepatic steatosis and fibrosis? Findings UGAP had a maximum AUC of 0.828 for the diagnosis of grade ≥ 2 steatosis, and 2D-SWE had an AUC of 0.862 for diagnosing advanced fibrosis. Clinical relevance UGAP and 2D-SWE allow rapid, reproducible, and accurate quantification of hepatic steatosis and fibrosis that can be used for the noninvasive assessment of patients with metabolic dysfunction-associated steatotic liver disease.
Keywords: Fatty liver; Fibrosis; Nonalcoholic fatty liver disease; Obesity; Ultrasonography.
© 2024. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Guarantor: The scientific guarantor of this publication is T.V.B. Conflict of interest: The authors of this manuscript declare relationships with the following companies: G.P. is a member of the Scientific Editorial Board (section: Oncology) for European Radiology. They did not participate in the selection or review processes for this article. R.C.: support for attending meetings from Bracco and Bayer; research collaboration with Siemens Healthineers. Statistics and biometry: One of the authors (R.C.) has significant statistical expertise. Informed consent: Written informed consent was obtained from all subjects (patients) in this study. Ethical approval: Institutional Review Board approval was obtained. Study subjects or cohorts overlap: None of the subjects or cohorts have been previously reported. Preliminary data reported in this manuscript were presented at SIRM 2022, ECR 2023, and ESGAR 2023 meetings. Methodology: Prospective Diagnostic or prognostic study Performed at one institution
Figures



References
-
- Rinella ME, Lazarus JV, Ratziu V et al (2023) A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 79:1542–1556. 10.1016/j.jhep.2023.06.003 - PubMed
-
- Ekstedt M, Hagström H, Nasr P et al (2015) Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61:1547–1554. 10.1002/hep.27368 - PubMed
-
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) (2016) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64:1388–1402. 10.1016/j.jhep.2015.11.004 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

