Evaluating a motor progression connectivity model across Parkinson's disease stages
- PMID: 39373780
- PMCID: PMC11561123
- DOI: 10.1007/s00415-024-12703-8
Evaluating a motor progression connectivity model across Parkinson's disease stages
Abstract
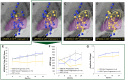
Background: Stimulation of a specific site in the dorsolateral subthalamic nucleus (STN) was recently associated with slower motor progression in Parkinson's Disease (PD), based on the deep brain stimulation (DBS) in early-stage PD pilot clinical trial. Here, subject-level visualizations are presented of this early-stage PD dataset to further describe the relationship between active contacts and motor progression. This study also evaluates whether stimulation of the sweet spot and connectivity model associated with slower motor progression is also associated with improvements in long-term motor outcomes in patients with advanced-stage PD.
Methods: Active contacts of the early-stage PD cohort (N = 14) were analyzed alongside the degree of two-year motor progression. Sweet spot and connectivity models derived from the early-stage PD cohort were then used to determine how well they can estimate the variance in long-term motor outcomes in an independent STN-DBS cohort of advanced-stage PD patients (N = 29).
Results: In early-stage PD, proximity of stimulation to the dorsolateral STN was associated with slower motor progression. In advanced-stage PD, stimulation proximity to the early PD connectivity model and sweet spot were associated with better long-term motor outcomes (R = 0.60, P < 0.001; R = 0.37, P = 0.046, respectively).
Conclusions: Results suggest stimulation of a specific site in the dorsolateral STN is associated with both slower motor progression and long-term motor improvements in PD.
Keywords: Deep brain stimulation; Motor symptoms; Movement disorders; Parkinson’s disease; Subthalamic nucleus.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

