Trojan-Horse Strategy Targeting the Gut-Liver Axis Modulates Gut Microbiome and Reshapes Microenvironment for Orthotopic Hepatocellular Carcinoma Therapy
- PMID: 39373804
- PMCID: PMC11600211
- DOI: 10.1002/advs.202310002
Trojan-Horse Strategy Targeting the Gut-Liver Axis Modulates Gut Microbiome and Reshapes Microenvironment for Orthotopic Hepatocellular Carcinoma Therapy
Abstract
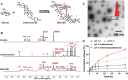
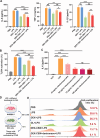
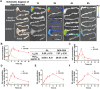
Reversing the hepatic inflammatory and immunosuppressive microenvironment caused by gut microbiota-derived lipopolysaccharides (LPS), accumulating to the liver through the gut-liver axis, is crucial for suppressing hepatocellular carcinoma (HCC) and metastasis. However, synergistically manipulating LPS-induced inflammation and gut microbiota remains a daunting task. Herein, a Trojan-horse strategy is proposed using an oral dextran-carbenoxolone (DEX-CBX) conjugate, which combines prebiotic and glycyrrhetinic acid (GA) homologs, to targeted delivery GA to HCC through the gut-liver axis for simultaneous modulation of hepatic inflammation and gut microbiota. In the orthotopic HCC model, a 95-45% reduction in the relative abundances of LPS-associated microbiota is observed, especially Helicobacter, caused by DEX-CBX treatment over phosphate-buffered saline (PBS) treatment. Notably, a dramatic increase (37-fold over PBS) in the abundance of Akkermansia, which is known to strengthen systemic immune response, is detected. Furthermore, DEX-CBX significantly increased natural killer T cells (5.7-fold) and CD8+ T cells (3.9-fold) as well as decreased M2 macrophages (59% reduction) over PBS treatment, resulting in a tumor suppression rate of 85.4%. DEX-CBX is anticipated to offer a novel strategy to precisely modulate hepatic inflammation and the gut microbiota to address both the symptoms and root causes of LPS-induced immunosuppression in HCC.
Keywords: gut microbiota; hepatic inflammation; hepatocellular carcinoma; immunotherapy; oral nanomedicine.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Filliol A., Schwabe R. F., Semin. Liver Dis. 2019, 39, 315; - PubMed
- b) Singal A. G., El‐Serag H. B., Clin. Gastroenterol. Hepatol. 2015, 13, 2140; - PMC - PubMed
- c) Ji G., Ma L., Yao H., Ma S., Si X., Wang Y., Bao X., Ma L., Chen F., Ma C., Huang L., Fang X., Song W., Acta Pharm. Sin. B 2020, 10, 2171. - PMC - PubMed
-
- Prieto J., Melero I., Sangro B., Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681. - PubMed
MeSH terms
Substances
Grants and funding
- 52103194/National Natural Science Foundation of China
- 51973215/National Natural Science Foundation of China
- 22105199/National Natural Science Foundation of China
- 20210504001GH/Jilin Province Science and Technology Department Plan
- 172408GH010234983/Jilin Province Science and Technology Department Plan
- YDZJ202101ZYTS131/Jilin Province Science and Technology Department Plan
- 2021050849RQ/Jilin Province Science and Technology Department Plan
- 2021YFC2400603/National Key Research and Development Program of China
- BX20220337/National Postdoctoral Program for Innovative Talents
- QT202103/the Youth Talents Promotion Project of Jilin Province
- JDYY-DEP-2022010/First Hospital of Jilin University
- 2023BZ01/Jilin University Norman Bethune Research Program
- 45123031H013/Basic Research Projects of Jilin University
LinkOut - more resources
Full Text Sources
Medical
Research Materials
