Circulating Tumor Cell Count and Overall Survival in Patients With Metastatic Hormone-Sensitive Prostate Cancer
- PMID: 39374015
- PMCID: PMC11581504
- DOI: 10.1001/jamanetworkopen.2024.37871
Circulating Tumor Cell Count and Overall Survival in Patients With Metastatic Hormone-Sensitive Prostate Cancer
Abstract
Importance: In metastatic hormone-sensitive prostate cancer (mHSPC), new first-line combination therapies have enhanced overall survival (OS), but clinical outcomes for individual patients vary greatly and are difficult to predict. Peripheral blood circulating tumor cell (CTC) count is the most extensively validated prognostic liquid biomarker in metastatic castration-resistant prostate cancer (mCRPC), and recent studies have suggested that it may also be informative in mHSPC.
Objective: To examine the prognostic value of CTC count in men with mHSPC.
Design, setting, and participants: In this prognostic study, peripheral blood was drawn at registration (baseline) and at progression to mCRPC in the S1216 study (March 1, 2013, to July 15, 2017), a phase 3, prospective, randomized clinical trial in men with mHSPC. The CTCs were enumerated using a US Food and Drug Administration-cleared isolation platform. Counts were categorized as 0, 1 to 4, or 5 or more CTCs per 7.5 mL based on the prognostic value of these cut points in prior studies. The data analysis was performed between October 28, 2022, and June 15, 2023.
Exposure: Metastatic hormone-sensitive prostate cancer.
Main outcomes and measures: Circulating tumor cell count was evaluated for an association with 3 prespecified trial end points: OS, progression-free survival, and 7-month prostate-specific antigen, after adjusting for other baseline covariates using proportional hazards and logistic regression models.
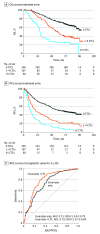
Results: Of 1313 S1216 participants (median [IQR] age, 68 [44-92] years), evaluable samples from 503 (median [IQR] age, 69 [46-90] years) with newly diagnosed mHSPC were collected at baseline, and 93 samples were collected at progression. Baseline counts were 5 or more CTCs per 7.5 mL in 60 samples (11.9%), 1 to 4 CTCs per 7.5 mL in 107 samples (21.3%), and 0 CTCs per 7.5 mL in 336 samples (66.8%). Median OS for men with 5 or more CTCs per 7.5 mL was 27.9 months (95% CI, 24.1-31.2 months) compared with 56.2 months (95% CI, 45.7-69.8 months) for men with 1 to 4 CTCs per 7.5 mL and not reached at 78.0 months follow-up for men with 0 CTCs per 7.5 mL. After adjusting for baseline clinical covariates, men with 5 or more CTCs per 7.5 mL at baseline had a significantly higher hazard of death (hazard ratio, 3.22; 95% CI, 2.22-4.68) and disease progression (hazard ratio, 2.46; 95% CI, 1.76-3.43) and a lower likelihood of prostate-specific antigen complete response (odds ratio, 0.26; 95% CI, 0.12-0.54) compared with men with 0 CTCs per 7.5 mL at baseline. Adding baseline CTC count to other known prognostic factors (covariates only: area under the curve, 0.73; 95% CI, 0.67-0.79) resulted in an increased prognostic value for 3-year survival (area under the curve, 0.79; 95% CI, 0.73-0.84).
Conclusions and relevance: In this prognostic study, the findings validate CTC count as a prognostic biomarker that improved upon existing prognostic factors and estimated vastly divergent survival outcomes regardless of subsequent lines of therapy. As such, baseline CTC count in mHSPC may serve as a valuable noninvasive biomarker to identify men likely to have poor survival who may benefit from clinical trials of intensified or novel regimens.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

