The role of single case experimental designs in evidence creation in rehabilitation
- PMID: 39374052
- PMCID: PMC11729724
- DOI: 10.23736/S1973-9087.24.08713-6
The role of single case experimental designs in evidence creation in rehabilitation
Abstract
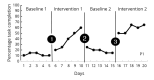
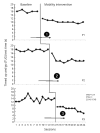
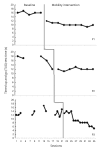
Randomized controlled trials (RCTs) are considered the gold standard of evidence guiding intervention selection in rehabilitation. However, conduct of sufficiently powered RCTs in rehabilitation can be expensive, pose ethical and attrition concerns when participants are assigned to ineffective treatment as usual conditions, and are infeasible with low-incidence populations. Single-case experimental designs (SCEDs), including N-of-1 RCTs are causal inference studies for small numbers of participants and not necessarily one participant as the name implies. These designs are increasingly used to evaluate the effectiveness of rehabilitation interventions in diverse clinical settings and employ design features including but not limited to randomization and each participant serving as their own control. These and other internal validity enhancements can increase the confidence in study results coming from these designs. This manuscript discusses the expanded application of SCEDs in rehabilitation contexts to answer everyday clinical rehabilitation research questions with emphasis on strategies to use: 1) to maximize internal validity of this family of designs; 2) improve utility, effectiveness, and acceptability of these designs for rehabilitation end-users (clinicians, policymakers, and participants); 3) build evidence bases in areas of rehabilitation where RCTs are uncommonly used. Primary considerations for situating SCEDs within the continuum of experimental designs include increasing internal validity within designs, improving transparency in conduct and reporting of these studies, and increasing access to advanced research methods training for rehabilitation professionals.
Conflict of interest statement
Figures
References
-
- Armijo-Olivo S, Mohamad N, Sobral de Oliveira-Souza AI, de Castro-Carletti EM, Ballenberger N, Fuentes J. Performance, detection, contamination, compliance, and cointervention biases in rehabilitation research: what are they and how can they affect the results of randomized controlled trials? Basic information for junior researchers and clinicians. Am J Phys Med Rehabil 2022;101:864–78. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1097/PHM.0000000000001893 - DOI - PubMed
-
- Dallery J, Raiff BR. Optimizing behavioral health interventions with single-case designs: from development to dissemination. Transl Behav Med 2014;4:290–303. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1007/s13142-014-0258-z - DOI - PMC - PubMed
-
- Krasny-Pacini A. Single-case experimental designs for child neurological rehabilitation and developmental disability research. Dev Med Child Neurol 2023;65:611–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1111/dmcn.15513 - DOI - PubMed
-
- Krasny-Pacini A, Evans J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Ann Phys Rehabil Med 2018;61:164–79. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1016/j.rehab.2017.12.002 - DOI - PubMed
-
- Selker HP, Cohen T, D’Agostino RB, Dere WH, Ghaemi SN, Honig PK, et al. A useful and sustainable role for N-of-1 trials in the healthcare ecosystem. Clin Pharmacol Ther 2022;112:224–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&l... 10.1002/cpt.2425 - DOI - PMC - PubMed