Overlap of spike and ripple propagation onset predicts surgical outcome in epilepsy
- PMID: 39374135
- PMCID: PMC11514932
- DOI: 10.1002/acn3.52156
Overlap of spike and ripple propagation onset predicts surgical outcome in epilepsy
Abstract
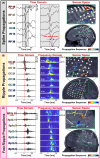
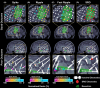
Objective: Interictal biomarkers are critical for identifying the epileptogenic focus. However, spikes and ripples lack specificity while fast ripples lack sensitivity. These biomarkers propagate from more epileptogenic onset to areas of spread. The pathophysiological mechanism of these propagations is elusive. Here, we examine zones where spikes and high frequency oscillations co-occur (SHFO), the spatiotemporal propagations of spikes, ripples, and fast ripples, and evaluate the spike-ripple onset overlap (SRO) as an epilepsy biomarker.
Methods: We retrospectively analyzed intracranial EEG data from 41 patients with drug-resistant epilepsy. We mapped propagations of spikes, ripples, and fast ripples, and identified their onset and spread zones, as well as SHFO and SRO. We then estimated the SRO prognostic value in predicting surgical outcome and compared it to onset and spread zones of spike, ripple, and fast ripple propagations, and SHFO.
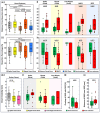
Results: We detected spikes and ripples in all patients and fast ripples in 12 patients (29%). We observed spike and ripple propagations in 40 (98%) patients. Spike and ripple onsets overlapped in 35 (85%) patients. In good outcome patients, SRO showed higher specificity and precision (p < 0.05) in predicting resection compared to onset and zones of spikes, ripples, and SHFO. Only SRO resection predicted outcome (p = 0.01) with positive and negative predictive values of 82% and 57%, respectively.
Interpretation: SRO is a specific and precise biomarker of the epileptogenic zone whose removal predicts outcome. SRO is present in most patients with drug-resistant epilepsy. Such a biomarker may reduce prolonged intracranial monitoring and improve outcome.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures


References
-
- Fujiwara H, Kadis DS, Greiner HM, et al. Clinical validation of magnetoencephalography network analysis for presurgical epilepsy evaluation. Clin Neurophysiol. 2022;142:199‐208. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
