Neuroprotective effects of punicalagin and/or micronized zeolite clinoptilolite on manganese-induced Parkinson's disease in a rat model: Involvement of multiple pathways
- PMID: 39374157
- PMCID: PMC11457879
- DOI: 10.1111/cns.70008
Neuroprotective effects of punicalagin and/or micronized zeolite clinoptilolite on manganese-induced Parkinson's disease in a rat model: Involvement of multiple pathways
Abstract
Background: Manganism, a central nervous system dysfunction correlated with neurological deficits such as Parkinsonism, is caused by the substantial collection of manganese chloride (MnCl2) in the brain.
Objectives: To explore the neuroprotective effects of natural compounds, namely, micronized zeolite clinoptilolite (ZC) and punicalagin (PUN), either individually or in combination, against MnCl2-induced Parkinson's disease (PD).
Methods: Fifty male albino rats were divided into 5 groups (Gps). Gp I was used as the control group, and the remaining animals received MnCl2 (Gp II-Gp V). Rats in Gps III and IV were treated with ZC and PUN, respectively. Gp V received both ZC and PUN as previously reported for the solo-treated plants.
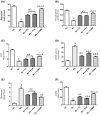
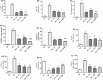
Results: ZC and/or PUN reversed the depletion of monoamines in the brain and decreased acetyl choline esterase activity, which primarily adjusted the animals' behavior and motor coordination. ZC and PUN restored the balance between glutamate/γ-amino butyric acid content and markedly improved the brain levels of brain-derived neurotrophic factor and nuclear factor erythroid 2-related factor 2/heme oxygenase-1 and decreased glycogen synthase kinase-3 beta activity. ZC and PUN also inhibited inflammatory and oxidative markers, including nuclear factor kappa-light-chain-enhancer of activated B cells, Toll-like receptor 4, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 and caspase-1. Bcl-2-associated X-protein and B-cell leukemia/lymphoma 2 protein (Bcl-2) can significantly modify caspase-3 expression. ZC and/or PUN ameliorated PD in rats by decreasing the levels of endoplasmic reticulum (ER) stress markers (p-protein kinase-like ER kinase (PERK), glucose-regulated protein 78, and C/EBP homologous protein (CHOP)) and enhancing the levels of an autophagy marker (Beclin-1).
Discussion and conclusion: ZC and/or PUN mitigated the progression of PD through their potential neurotrophic, neurogenic, anti-inflammatory, antioxidant, and anti-apoptotic activities and by controlling ER stress through modulation of the PERK/CHOP/Bcl-2 pathway.
Keywords: Parkinson's disease; antioxidant; anti‐inflammatory; autophagy; endoplasmic reticulum stress; micronized zeolite clinoptilolite; oxidative stress; punicalagin.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



References
-
- Kozieł S, Wojtala D, Szmitka M, Sawka J, Komarnicka UK. Can Mn coordination compounds be good candidates for medical applications? Front Chem Biol. 2024;3:1337372.
-
- Gajula S et al. Relative bioavailability of manganese from its chloride, oxide and sulphate salts for broiler chickens. Indian J Anim Sci. 2008;78:775‐779.
-
- Nadeem RI, Ahmed HI, El‐Sayeh BM. Protective effect of vinpocetine against neurotoxicity of manganese in adult male rats. Naunyn Schmiedeberg's Arch Pharmacol. 2018;391:729‐742. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

