A flexible generative algorithm for growing in silico placentas
- PMID: 39374295
- PMCID: PMC11486434
- DOI: 10.1371/journal.pcbi.1012470
A flexible generative algorithm for growing in silico placentas
Abstract
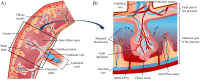
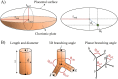
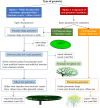
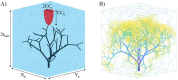
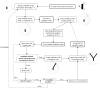
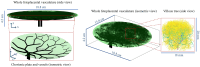
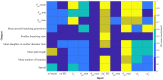
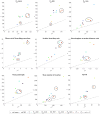
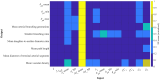
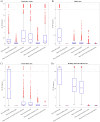
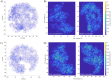
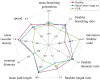
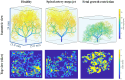
The placenta is crucial for a successful pregnancy, facilitating oxygen exchange and nutrient transport between mother and fetus. Complications like fetal growth restriction and pre-eclampsia are linked to placental vascular structure abnormalities, highlighting the need for early detection of placental health issues. Computational modelling offers insights into how vascular architecture correlates with flow and oxygenation in both healthy and dysfunctional placentas. These models use synthetic networks to represent the multiscale feto-placental vasculature, but current methods lack direct control over key morphological parameters like branching angles, essential for predicting placental dysfunction. We introduce a novel generative algorithm for creating in silico placentas, allowing user-controlled customisation of feto-placental vasculatures, both as individual components (placental shape, chorionic vessels, placentone) and as a complete structure. The algorithm is physiologically underpinned, following branching laws (i.e. Murray's Law), and is defined by four key morphometric statistics: vessel diameter, vessel length, branching angle and asymmetry. Our algorithm produces structures consistent with in vivo measurements and ex vivo observations. Our sensitivity analysis highlights how vessel length variations and branching angles play a pivotal role in defining the architecture of the placental vascular network. Moreover, our approach is stochastic in nature, yielding vascular structures with different topological metrics when imposing the same input settings. Unlike previous volume-filling algorithms, our approach allows direct control over key morphological parameters, generating vascular structures that closely resemble real vascular densities and allowing for the investigation of the impact of morphological parameters on placental function in upcoming studies.
Copyright: © 2024 C. de Oliveira et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

