Thrombectomy for Stroke With Large Infarct on Noncontrast CT: The TESLA Randomized Clinical Trial
- PMID: 39374319
- PMCID: PMC11420819
- DOI: 10.1001/jama.2024.13933
Thrombectomy for Stroke With Large Infarct on Noncontrast CT: The TESLA Randomized Clinical Trial
Abstract
Importance: Recent large infarct thrombectomy trials used heterogeneous imaging modalities and time windows for patient selection. Noncontrast computed tomographic (CT) scan is the most common stroke imaging approach. It remains uncertain whether thrombectomy is effective for patients with large infarcts identified using noncontrast CT alone within 24 hours of stroke onset.
Objective: To evaluate the effect of thrombectomy in patients with a large infarct on a noncontrast CT scan within 24 hours of onset.
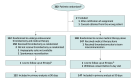
Design, setting, and participants: Open-label, blinded-end point, bayesian-adaptive randomized trial with interim analyses for early stopping (futility or success) or population enrichment, which was conducted at 47 US academic and community-based stroke thrombectomy centers. Three hundred patients presenting within 24 hours with anterior-circulation, large-vessel occlusion and large infarct on noncontrast CT scan, with Alberta Stroke Program Early CT Scores of 2 to 5, were randomized to undergo thrombectomy or usual care. Enrollment occurred July 16, 2019 to October 17, 2022; final follow-up, January 25, 2023.
Intervention: The intervention patients (n = 152) underwent endovascular treatment using standard thrombectomy devices and usual medical care. Control patients (n = 148) underwent usual medical care alone.
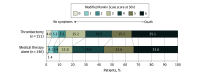
Main outcomes and measures: The primary efficacy end point was improvement in 90-day functional outcome measured using mean utility-weighted modified Rankin Scale (UW-mRS) scores (range, 0 [death or severe disability] to 10 [no symptoms]; minimum clinically important difference, 0.3). A bayesian model determined the posterior probability that the intervention would be superior to usual care; statistical significance was a 1-sided posterior probability of .975 or more. The primary adverse event end point was 90-day mortality; secondary adverse event end points included symptomatic intracranial hemorrhage and radiographic intracranial hemorrhage.
Results: The trial enrolled 300 patients (152 intervention, 148 control; 138 females [46%]; median age, 67 years), without early stopping or enrichment; 297 patients completed the 90-day follow-up. The mean (SD) 90-day UW-mRS score was 2.93 (3.39) for the intervention group vs 2.27 (2.98) for the control group with an adjusted difference of 0.63 (95% credible interval [CrI], -0.09 to 1.34; posterior probability for superiority of thrombectomy, .96). The 90-day mortality was similar between groups: 35.3% (53 of 150) for the intervention group vs 33.3% (49 of 147) for the control group. Six of 151 patients (4.0%) in the intervention group and 2 of 149 (1.3%) in the control group experienced 24-hour symptomatic intracranial hemorrhage. Fourteen patients of 148 (9.5%) in the intervention group vs 4 of 146 (2.7%) in the control group experienced parenchymal hematoma type 1 hemorrhages; 14 (9.5%) in the intervention group vs 5 (3.4%) in the control group experienced parenchymal hematoma type 2 hemorrhages; and 24 (16.2%) in the intervention group vs 9 (6.2%) in the control group experienced subarachnoid hemorrhages.
Conclusions and relevance: Among patients with a large infarct on noncontrast CT within 24 hours, thrombectomy did not demonstrate improvement in functional outcomes. But the width of the credible interval around the effect estimate includes the possibility of both no important effect and a clinically relevant benefit, so the potential role of thrombectomy with this imaging approach and time window will likely require additional study.
Trial registration: ClinicalTrials.gov Identifier: NCT03805308.
Conflict of interest statement
Figures
Comment in
References
-
- Yoo AJ, Berkhemer OA, Fransen PSS, et al. ; MR CLEAN investigators . Effect of baseline Alberta Stroke Program Early CT Score on safety and efficacy of intra-arterial treatment: a subgroup analysis of a randomised phase 3 trial (MR CLEAN). Lancet Neurol. 2016;15(7):685-694. doi: 10.1016/S1474-4422(16)00124-1 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

