Neurotransmitter release is triggered by a calcium-induced rearrangement in the Synaptotagmin-1/SNARE complex primary interface
- PMID: 39374398
- PMCID: PMC11494337
- DOI: 10.1073/pnas.2409636121
Neurotransmitter release is triggered by a calcium-induced rearrangement in the Synaptotagmin-1/SNARE complex primary interface
Abstract
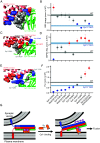
The Ca2+ sensor synaptotagmin-1 (Syt1) triggers neurotransmitter release together with the neuronal sensitive factor attachment protein receptor (SNARE) complex formed by syntaxin-1, SNAP25, and synaptobrevin. Moreover, Syt1 increases synaptic vesicle (SV) priming and impairs spontaneous vesicle release. The Syt1 C2B domain binds to the SNARE complex through a primary interface via two regions (I and II), but how exactly this interface mediates distinct functions of Syt1 and the mechanism underlying Ca2+ triggering of release are unknown. Using mutagenesis and electrophysiological experiments, we show that region II is functionally and spatially subdivided: Binding of C2B domain arginines to SNAP-25 acidic residues at one face of region II is crucial for Ca2+-evoked release but not for vesicle priming or clamping of spontaneous release, whereas other SNAP-25 and syntaxin-1 acidic residues at the other face mediate priming and clamping of spontaneous release but not evoked release. Mutations that disrupt region I impair the priming and clamping functions of Syt1 while, strikingly, mutations that enhance binding through this region increase vesicle priming and clamping of spontaneous release, but strongly inhibit evoked release and vesicle fusogenicity. These results support previous findings that the primary interface mediates the functions of Syt1 in vesicle priming and clamping of spontaneous release and, importantly, show that Ca2+ triggering of release requires a rearrangement of the primary interface involving dissociation of region I, while region II remains bound. Together with biophysical studies presented in [K. Jaczynska et al., bioRxiv [Preprint] (2024). https://doi.org/10.1101/2024.06.17.599417 (Accessed 18 June 2024)], our data suggest a model whereby this rearrangement pulls the SNARE complex to facilitate fast SV fusion.
Keywords: SNAREs; neurotransmitter release; structure–function analysis; synaptic vesicle fusion; synaptotagmin.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





Update of
-
Neurotransmitter release is triggered by a calcium-induced rearrangement in the Synaptotagmin-1/SNARE complex primary interface.bioRxiv [Preprint]. 2024 Jun 21:2024.06.17.599435. doi: 10.1101/2024.06.17.599435. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2409636121. doi: 10.1073/pnas.2409636121. PMID: 38948868 Free PMC article. Updated. Preprint.
References
-
- Sutton R. B., Fasshauer D., Jahn R., Brunger A. T., Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

