Microstructural Characterization of Dry Powder Inhaler Formulations Using Orthogonal Analytical Techniques
- PMID: 39375241
- PMCID: PMC11530509
- DOI: 10.1007/s11095-024-03776-1
Microstructural Characterization of Dry Powder Inhaler Formulations Using Orthogonal Analytical Techniques
Abstract
Purpose: For locally-acting dry powder inhalers (DPIs), developing novel analytical tools that are able to evaluate the state of aggregation may provide a better understanding of the impact of material properties and processing parameters on the in vivo performance. This study explored the utility of the Morphologically-Directed Raman Spectroscopy (MDRS) and dissolution as orthogonal techniques to assess microstructural equivalence of the aerosolized dose of DPIs collected with an aerosol collection device.
Methods: Commercial DPIs containing different strengths of Fluticasone Propionate (FP) and Salmeterol Xinafoate (SX) as monotherapy and combination products were sourced from different regions. These inhalers were compared with aerodynamic particle size distribution (APSD), dissolution, and MDRS studies.
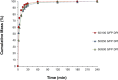
Results: APSD testing alone might not be able to explain differences reported elsewhere in in vivo studies of commercial FP/SX drug products with different Advair® strengths and/or batches. Dissolution studies demonstrated different dissolution rates between Seretide™ 100/50 and Advair® 100/50, whereas Flixotide™ 100 and Flovent® 100 had similar dissolution rates between each other. These differences in dissolution profiles were supported by MDRS results: the dissolution rate is increased if the fraction of FP associated with high soluble components is increased. Principle component analysis was used to identify the agglomerate classes that better discriminate different products.
Conclusions: MDRS and dissolution studies of the aerosolized dose of DPIs were successfully used as orthogonal techniques. This study highlights the importance of further assessing in vitro tools that are able to provide a bridge between material attributes or process parameters and in vivo performance.
Keywords: bioequivalence; dissolution; dry powder inhaler; orthogonal analytical techniques; raman spectroscopy.
© 2024. The Author(s).
Conflict of interest statement
Views expressed in this article are from the authors and do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Figures









References
-
- US Food and Drug Administration. Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Products - Quality Considerations Guidance for Industry Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Products - Quality Considerations Guidance for Industry Contains Nonbinding Recommendat [Internet]. Online. 2018 [cited 2020 Apr 26]. Available from: https://www.fda.gov/media/70851/download
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

