Natural hydroxyapatite-based nanobiocomposites and their biomaterials-to-cell interaction for bone tissue engineering
- PMID: 39375246
- PMCID: PMC11458869
- DOI: 10.1186/s11671-024-04119-0
Natural hydroxyapatite-based nanobiocomposites and their biomaterials-to-cell interaction for bone tissue engineering
Abstract
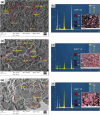
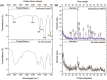
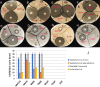
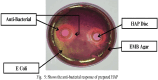
Hydroxyapatite (HA) is an extensively used biomaterial for dental and orthopaedic applications because of its biocompatibility and biomimetic nature. HA is extensively used as a bone-graft substitute. HA bone graft substitutes of bovine or synthetic origins have been extensively studied. However, caprine-based HA has not been explored. In this study, we aimed to determine the utilization of goat bone-derived HA for commercial applications. HA from caprine bone and teeth was isolated using thermal calcination. The developed HA can be used as a bone graft substitute. Chemical characterization of the isolated HA was carried out using Fourier transform infrared spectroscopy, X-Ray Diffraction, Raman spectroscopy, scanning electron microscopy, and transmission electron microscopy. The biocompatibility and apatite formation of isolated HA were assessed using MG-63 cells, MC3T3-E1, L929 cells, MSCs, adipose derived stem cells, human dermal tissue derived fibroblast cells and osteoblast-like cell line, The studies demonstrate that HA support cell adhesion and osteogenic properties. To improve sheep, lamp, or caprine bone-derived HA, several other composites have been developed with MgO2, ZrO2, ZnO2, and other polymeric substances. 3D printed technology was used to develop a bioink using sheep-derived HA and printed the composite scaffold as a bone graft substitute. Furthermore, the biomedical applications of sheep-derived HA been studied in terms of their antimicrobial activity, bone-forming ability, and wound healing applications. Sheep-, goat-, and caprine-derived HA are still underutilized and require further research to develop commercial possibilities and sustainable raw materials for HA-based bone graft substitutes.
Keywords: Bone-graft substitute; Caprine bone; Goat bone; Sheep bone; Thermal calcination.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Irfan M, Irfan M. Overview of hydroxyapatite; composition, structure, synthesis methods and its biomedical uses. Biomed Lett. 2020;6:17–22.
-
- Afshar A, Ghorbani M, Ehsani N, Saeri M, Sorrell C. Some important factors in the wet precipitation process of hydroxyapatite. Mater Des. 2003;24:197–202. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
