Brain Abnormalities in Young Single- and Double-Heterozygote Mice for Both Nkx2-1- and Pax8-Null Mutations
- PMID: 39375286
- PMCID: PMC11880048
- DOI: 10.1007/s12035-024-04524-7
Brain Abnormalities in Young Single- and Double-Heterozygote Mice for Both Nkx2-1- and Pax8-Null Mutations
Abstract
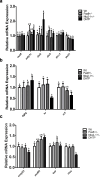
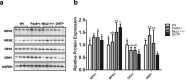
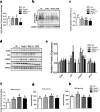
In humans and mice, Nkx2-1 and Pax8 are crucial morphogenic transcription factors defining the early development of the thyroid and specific extrathyroidal tissues. By using 3-month-old single or double heterozygotes for Nkx2-1- and Pax8-null mutations (DHTP) mice, we studied brain abnormalities under different human-like dysthyroidisms, focusing on putative alterations of specific neurotransmitter systems, expression of markers of pre- and post-synaptic function and, given the physio-pathological role mitochondria have in controlling the bioenergetic status of neurons, of mitochondrial dynamics and oxidative balance. Compared to Wt controls, DHTP mice, bearing both systemic and brain hypothyroidism, showed altered expression of synaptic markers, generic and cholinergic (corroborated by immunohistochemistry in caudate, putamen, hippocampus, and basal forebrain) and glutamatergic ones, and reduced expression of key proteins of synaptic plasticity potency and several isoforms of glutamate receptors. The brain of DHTP mice was characterized by lower levels of H2O2 and imbalanced mitochondrial dynamics. Nkx2-1 + / - mice showed dopaminergic neuron-specific alterations, morphologically, more evident in the substantia nigra of DHTP mice. Nkx2-1 + / - mice also showed enhanced mitochondrial biogenesis and oxidative capacity likely as a global response of the brain to Nkx2-1 haploinsufficiency and/or to their elevated T3 circulating levels. Reduced transcription of both tyrosine hydroxylase and dopamine transporter was observed in Pax8 + / - euthyroid mice, suggesting a dopaminergic dysfunction, albeit likely at an early stage, but consistent with the deregulated glucose homeostasis observed in such animals. Overall, new information was obtained on the impact of haploinsufficiency of Pax8 and NKx2-1 on several brain neuroanatomical, molecular, and neurochemical aspects, thus opening the way for future targeting brain dysfunctions in the management of both overt and subclinical thyroid dysfunctions.
Keywords: Congenital hypothyroidism; Mitochondria; Oxidative stress; Synapses.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: Mouse protocols were approved by the Ethics Committee of Biogem (IRGS, Via Camporeale, 83031 Ariano Irpino, Avellino, Italy). All experiments were performed in accordance with the guidelines approved by the Italian Ministry of Health (project authorization number, 518/2016-PR). Consent to Participate: Not applicable. Consent for Publication: Not applicable. Competing Interests: The authors declare no competing interests.
Figures





References
-
- Churilov LP, Stroev, YI (2019) The tale of the knights of the shield: history of ideas in thyroidology. In: Churilov LP, Stroev YI, Akhmanov MS (eds) Essays on the history of medicine. Biographical sketches, 3rd edn. Umnyi Doktor, Moscow, pp 71–300 [in Russian]
-
- Bernal J (2022) Thyroid hormones in brain development and function. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (eds). Endotext [Internet]. MDText.com, Inc, South Dartmouth (MA), pp 2000–Available from: https://www.ncbi.nlm.nih.gov/books/NBK285549/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

