Boosting selective Cs+ uptake through the modulation of stacking modes in layered niobate-based perovskites
- PMID: 39375328
- PMCID: PMC11458626
- DOI: 10.1038/s41467-024-52920-3
Boosting selective Cs+ uptake through the modulation of stacking modes in layered niobate-based perovskites
Abstract
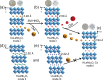
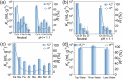
Selective separation of 137Cs is significant for the sustainable development of nuclear energy and environmental protection, due to its strong radioactivity and long half-life. However, selective capture of 137Cs+ from radioactive liquid waste is challenging due to strong coulomb interactions between the adsorbents and high-valency metal ions. Herein, we propose a strategy to resolve this issue and achieve specific Cs+ ion recognition and separation by modulating the stacking modes of layered perovskites. We demonstrate that among niobate-based perovskites, ALaNb2O7 (A = Cs, H, K, and Li), HLaNb2O7 shows an outstanding selectivity for Cs+ even in the presence of a large amount of competing Mn+ ions (Mn+ = K+, Ca2+, Mg2+, Sr2+, Eu3+, and Zr4+) owing to its suitable void fraction and space shape, brought by the stacking mode of layers. The Cs+ capture mechanism is directly elucidated at molecular level by single-crystal structural analyses and density functional theory calculations. This work not only provides key insights in the design and property optimization of perovskite-type materials for radiocesium separation, but also paves the way for the development of efficient inorganic materials for radionuclides remediation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Chen, S. et al. A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade. Sep. Sci. Technol.251, 117340 (2020).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

