Actin-driven nanotopography promotes stable integrin adhesion formation in developing tissue
- PMID: 39375335
- PMCID: PMC11458790
- DOI: 10.1038/s41467-024-52899-x
Actin-driven nanotopography promotes stable integrin adhesion formation in developing tissue
Abstract
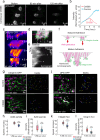
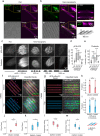
Morphogenesis requires building stable macromolecular structures from highly dynamic proteins. Muscles are anchored by long-lasting integrin adhesions to resist contractile force. However, the mechanisms governing integrin diffusion, immobilization, and activation within developing tissues remain elusive. Here, we show that actin polymerization-driven membrane protrusions form nanotopographies that enable strong adhesion at Drosophila muscle attachment sites (MASs). Super-resolution microscopy reveals that integrins assemble adhesive belts around Arp2/3-dependent actin protrusions, forming invadosome-like structures with membrane nanotopographies. Single protein tracking shows that, during MAS development, integrins become immobile and confined within diffusion traps formed by the membrane nanotopographies. Actin filaments also display restricted motion and confinement, indicating strong mechanical connection with integrins. Using isolated muscle cells, we show that substrate nanotopography, rather than rigidity, drives adhesion maturation by regulating actin protrusion, integrin diffusion and immobilization. These results thus demonstrate that actin-polymerization-driven membrane protrusions are essential for the formation of strong integrin adhesions sites in the developing embryo, and highlight the important contribution of geometry to morphogenesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

