Two-dimensional coordination risedronate-manganese nanobelts as adjuvant for cancer radiotherapy and immunotherapy
- PMID: 39375342
- PMCID: PMC11458765
- DOI: 10.1038/s41467-024-53084-w
Two-dimensional coordination risedronate-manganese nanobelts as adjuvant for cancer radiotherapy and immunotherapy
Abstract
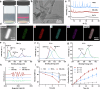
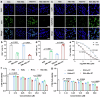
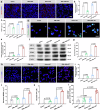
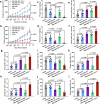
The irradiated tumor itself represents an opportunity to establish endogenous in situ vaccines. However, such in situ cancer vaccination (ISCV) triggered by radiation therapy (RT) alone is very weak and hardly elicits systemic anticancer immunity. In this study, we develop two-dimensional risedronate-manganese nanobelts (RMn-NBs) as an adjuvant for RT to address this issue. RMn-NBs exhibit good T2 magnetic resonance imaging performance and enhanced Fenton-like catalytic activity, which induces immunogenic cell death. RMn-NBs can inhibit the HIF-1α/VEGF axis to empower RT and synchronously activate the cGAS/STING pathway for promoting the secretion of type I interferon, thereby boosting RT-triggered ISCV and immune checkpoint blockade therapy against primary and metastatic tumors. RMn-NBs as a nano-adjuvant for RT show good biocompatibility and therapeutic efficacy, presenting a promising prospect for cancer radiotherapy and immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

