Compensatory activity of the PC-ME1 metabolic axis underlies differential sensitivity to mitochondrial complex I inhibition
- PMID: 39375345
- PMCID: PMC11458614
- DOI: 10.1038/s41467-024-52968-1
Compensatory activity of the PC-ME1 metabolic axis underlies differential sensitivity to mitochondrial complex I inhibition
Abstract
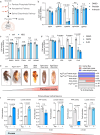
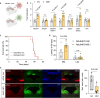
Deficiencies in the electron transport chain (ETC) lead to mitochondrial diseases. While mutations are distributed across the organism, cell and tissue sensitivity to ETC disruption varies, and the molecular mechanisms underlying this variability remain poorly understood. Here we show that, upon ETC inhibition, a non-canonical tricarboxylic acid (TCA) cycle upregulates to maintain malate levels and concomitant production of NADPH. Our findings indicate that the adverse effects observed upon CI inhibition primarily stem from reduced NADPH levels, rather than ATP depletion. Furthermore, we find that Pyruvate carboxylase (PC) and ME1, the key mediators orchestrating this metabolic reprogramming, are selectively expressed in astrocytes compared to neurons and underlie their differential sensitivity to ETC inhibition. Augmenting ME1 levels in the brain alleviates neuroinflammation and corrects motor function and coordination in a preclinical mouse model of CI deficiency. These studies may explain why different brain cells vary in their sensitivity to ETC inhibition, which could impact mitochondrial disease management.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. We support inclusive, diverse, and equitable conduct of research.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

